When you talk π-aromaticity, benzene is the first molecule that springs to mind. But there are smaller molecules that can carry this property; cyclopropenylidene (five atoms) is the smallest in terms of atom count I could think of until now, apart that is from H3+ which is the smallest possible molecule that carries σ-aromaticity.
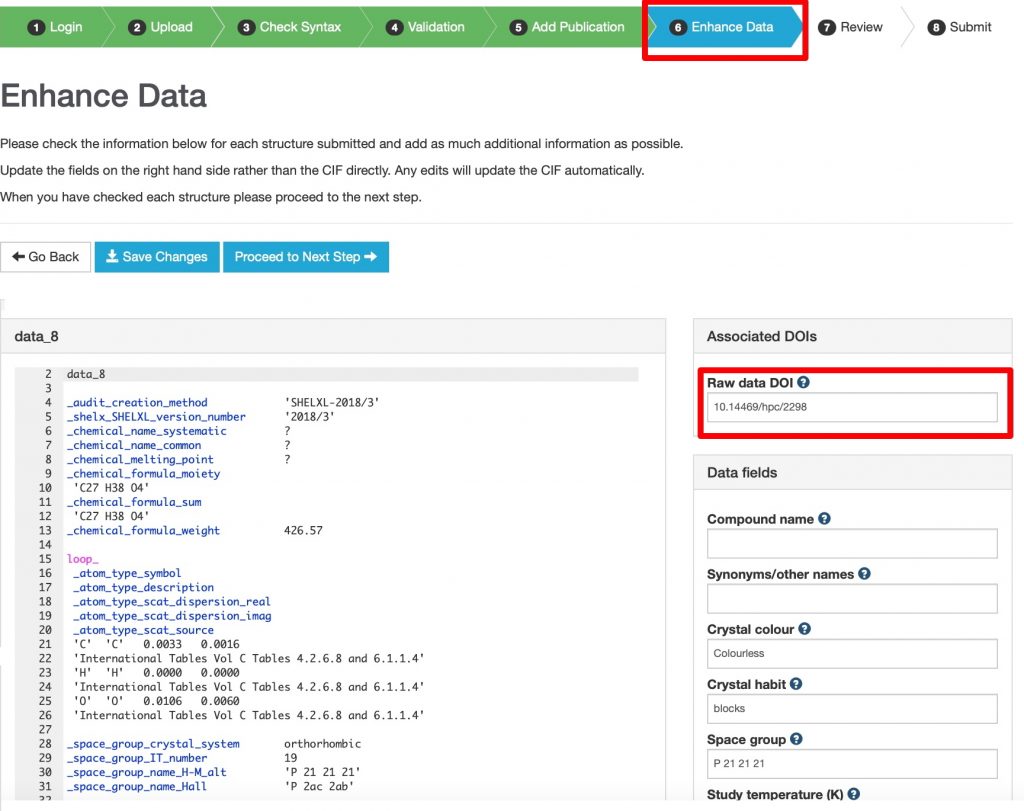
Scientific data in chemistry has come a long way in the last few decades. Originally entangled into scientific articles in the form of tables of numbers or diagrams, it was (partially) disentangled into supporting information when journals became electronic in the late 1990s.
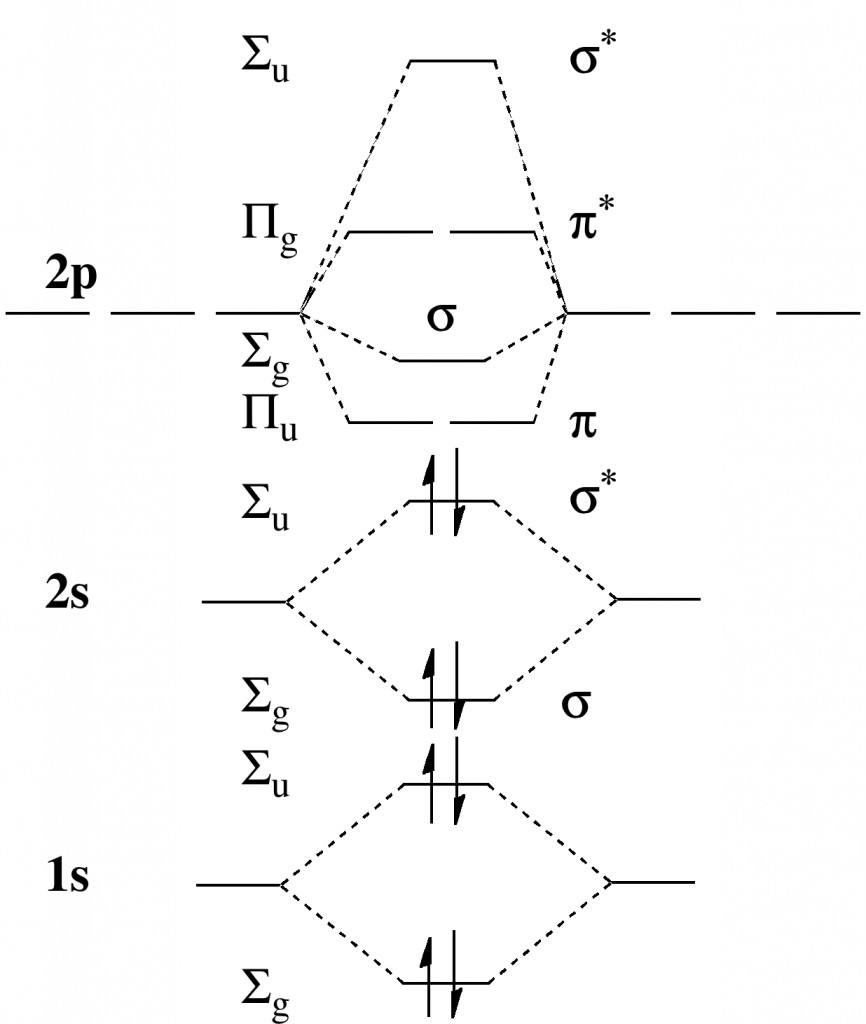
Ever since the concept of a shared two-electron bond was conjured by Gilbert N. Lewis in 1916, chemists have been fascinated by the related concept of a bond order (the number of such bonds that two atoms can participate in, however a bond is defined) and pushing it ever higher for pairs of like-atoms.
Way back in the late 1980s or so, research groups in chemistry started to replace the filing of their paper-based research data by storing it in an easily retrievable digital form. This required a computer database and initially these were accessible only on specific dedicated computers in the laboratory.
To be FAIR, data has to be not only Findable and Accessible, but straightforwardly Interoperable. One of the best examples of interoperability in chemistry comes from the domain of quantum chemistry. This strives to describe a molecule by its electron density distribution, from which many interesting properties can then be computed.
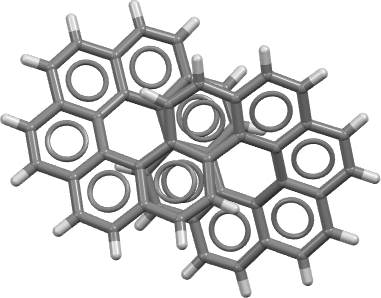
The annual “molecule of the year” results for 2021 are now available … and the winner is Infinitene., This is a benzocirculene in the form of a figure eight loop (the infinity symbol), a shape which is also called a lemniscate after the mathematical (2D) function due to Bernoulli.
In the previous post, I showed some of the diverse “non-classical”interactions between Biotin and a protein where it binds very strongly. Here I take a look at two of these interactions to discover how common they are in small molecule structures.
The title comes from the abstract of an article analysing why Biotin (vitamin B7) is such a strong and effective binder to proteins, with a free energy of (non-covalent) binding approaching 21 kcal/mol.
Earlier this year, Molnupiravir hit the headlines as a promising antiviral drug.
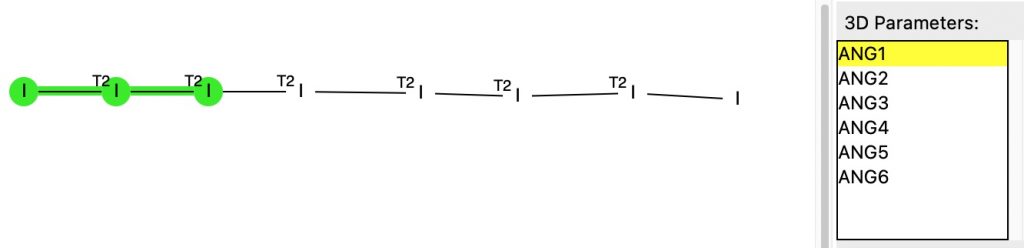
The previous post described the fascinating 170-year history of a crystalline compound known as Herapathite and its connection to the mechanism of the Finkelstein reaction via the complex of Na+I2– (or Na22+I42-). Both compounds exhibit (approximately) linear chains of iodine atoms in their crystal structures, a connection which was discovered serendipitously.
On October 13, 2021, the historical group of the Royal Society of Chemistry organised a symposium celebrating ~150 years of the history of (molecular) chirality. We met for the first time in person for more than 18 months and were treated to a splendid and diverse program about the subject.