I occasionally notice that posts that first appeared here many years ago suddenly attract attention.
Earlier, I explored the choreography or “timing”, of what might be described as the curly arrows for a typical taught reaction mechanism, the 1,4-addition of a nucleophile to an unsaturated carbonyl compound (scheme 1). I am now going to explore the consequences of changing one of the actors by adding the nucleophile to an unsaturated imine rather than carbonyl compound (scheme 2). **
A little more than a year ago, a ChemRxiv pre-print appeared bearing the title referenced in this post,[cite]10.26434/chemrxiv.8009633.v1[/cite] which immediately piqued my curiosity. The report presented persuasive evidence, in the form of trapping experiments, that dicarbon or C 2 had been formed by the following chemical synthesis.
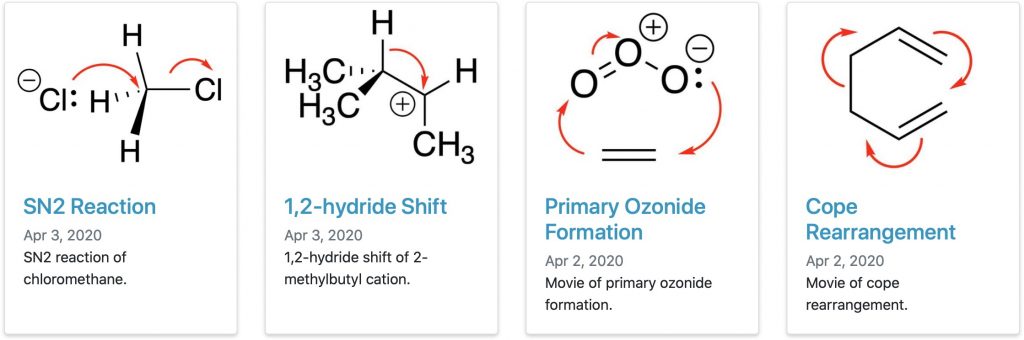
In a previous post, I talked about a library of reaction pathway intrinsic reaction coordinates (IRCs) containing 115 examples of organic and organometallic reactions. Now (thanks Dean!) I have been alerted to a brand new databank of dynamics trajectories (DDT), with the focus on those reactions taught in undergraduate organic chemistry courses, some of which are shown below.
In the news this week is a report of a molecule whose crystal lattice is capable of both storing and releasing large amounts of hydrogen gas at modest pressures and temperatures. Thus “NU-1501-Al” can absorb 14 weight% of hydrogen. To power a low-polluting car with a 500 km range, about 4-5 kg of hydrogen gas would be need to be stored and released safely.
A reaction can be thought of as molecular dancers performing moves. A choreographer is needed to organise the performance into the ballet that is a reaction mechanism. Here I explore another facet of the Michael addition of a nucleophile to a conjugated carbonyl compound.
In the previous post, I introduced three of a new generation of search engines specialising in the discovery of data. Data has some special features which make its properties slightly different from the conceptual (or natural language) searches we are used to performing for general information and so a search engine specifically for data is invariably going to reflect this.
Chemists have long been familiar with search engines that aspire to index a large proportion of the chemical literature.
In the previous post, I looked at the mechanism for 1,4-nucleophilic addition to an activated alkene (the Michael reaction). The model nucleophile was malonaldehyde after deprotonation and the model electrophile was acrolein (prop-2-enal), with the rate determining transition state being carbon-carbon bond formation between the two, accompanied by proton transfer to the oxygen of the acrolein.
In 2013, I created an iTunesU library of 115 mechanistic types in organic and organometallic chemistry, illustrated using video animations of the intrinsic reaction coordinate (IRC) computed using a high level quantum mechanical procedure. Many of those examples first derived from posts here. That collection is still available and is viewable in the iTunesU app on an iPhone or an iPad.
