Here is the concluding part of my exploration of a recently published laboratory experiment for undergraduate students. I had previously outlined a possible mechanistic route, identifying TS3 (below) as the first transition state in which C-C bond formation creates two chiral centres.
Recently, the 100th anniversary of the birth of the famous chemist Derek Barton was celebrated with a symposium.
I am exploring the fascinating diverse facets of a recently published laboratory experiment for undergraduate students. Previously I looked at a possible mechanistic route for the reaction between an enal (a conjugated aldehyde-alkene) and benzyl chloride catalysed by base and a chiral amine, followed by the use of NMR coupling constants to assign relative stereochemistries.
In the previous post, I investigated the mechanism of cyclopropanation of an enal using a benzylic chloride using a quantum chemistry based procedure. Here I take a look at the NMR spectra of the resulting cyclopropane products, with an evaluation of the original stereochemical assignments.
Symbiosis between computation and experiment is increasingly evident in pedagogic journals such as J. Chemical Education. Thus an example of original laboratory experiments, that later became twinned with a computational counterpart. So when I spotted this recent lab experiment I felt another twinning approaching.

Following the general recognition of carbon as being tetrahedrally tetravalent in 1869 (Paterno) and 1874 (Van’t Hoff and Le Bell), an early seminal exploitation of this to the conformation of cyclohexane was by Hermann Sachse in 1890.

In 2012, I wrote a story of the first ever reaction curly arrows, attributed to Robert Robinson in 1924. At the time there was a great rivalry between him and another UK chemist, Christopher Ingold, with the latter also asserting his claim for their use.
White City is a small area in west london created as an exhibition site in 1908, morphing over the years into an Olympic games venue, a greyhound track, the home nearby of the BBC (British Broadcasting Corporation) and most recently the new western campus for Imperial College London.♣ The first Imperial department to move into […]
Harnessing FAIR data is an event being held in London on September 3rd; no doubt all the speakers will espouse its virtues and speculate about how to realize its potential.♥ Admirable aspirations indeed. Capturing hearts and minds also needs lots of real life applications!
Consider the four reactions.
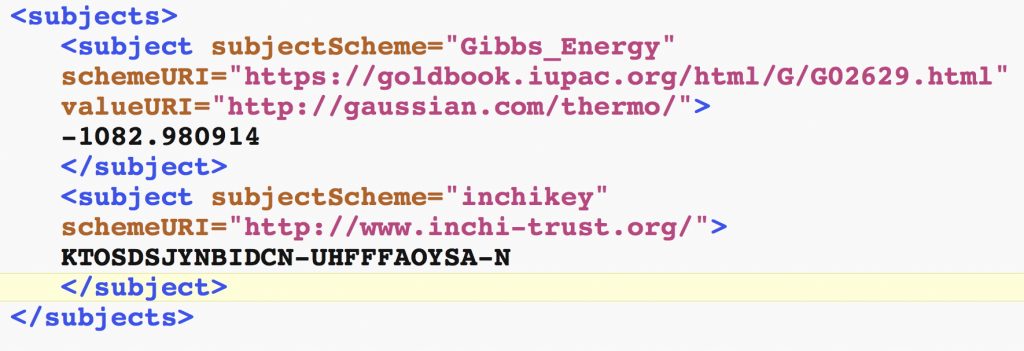
FAIR is one of those acronyms that spreads rapidly, acquires a life of its own and can mean many things to different groups. A two-day event has just been held in Amsterdam to bring some of those groups from the chemical sciences together to better understand FAIR.