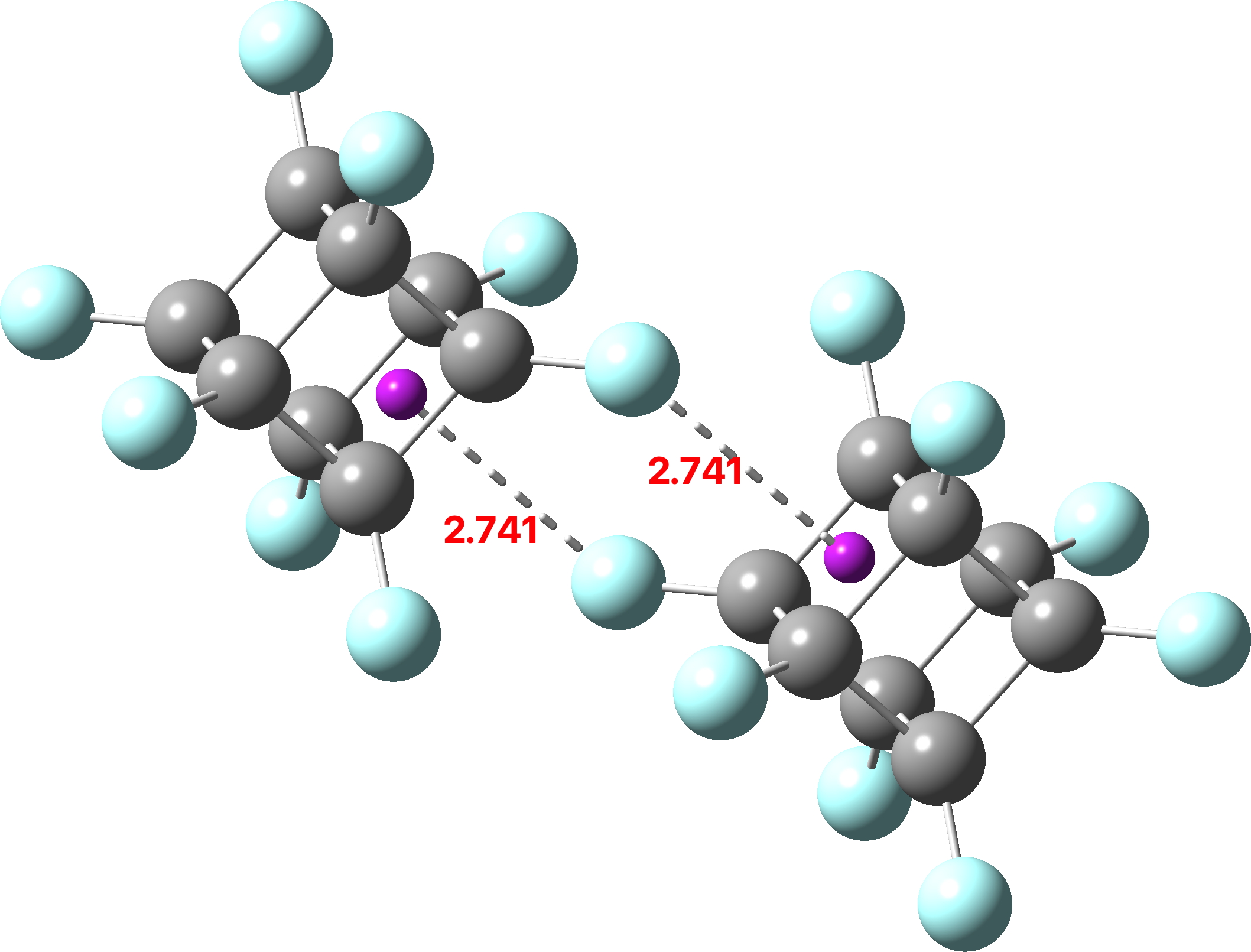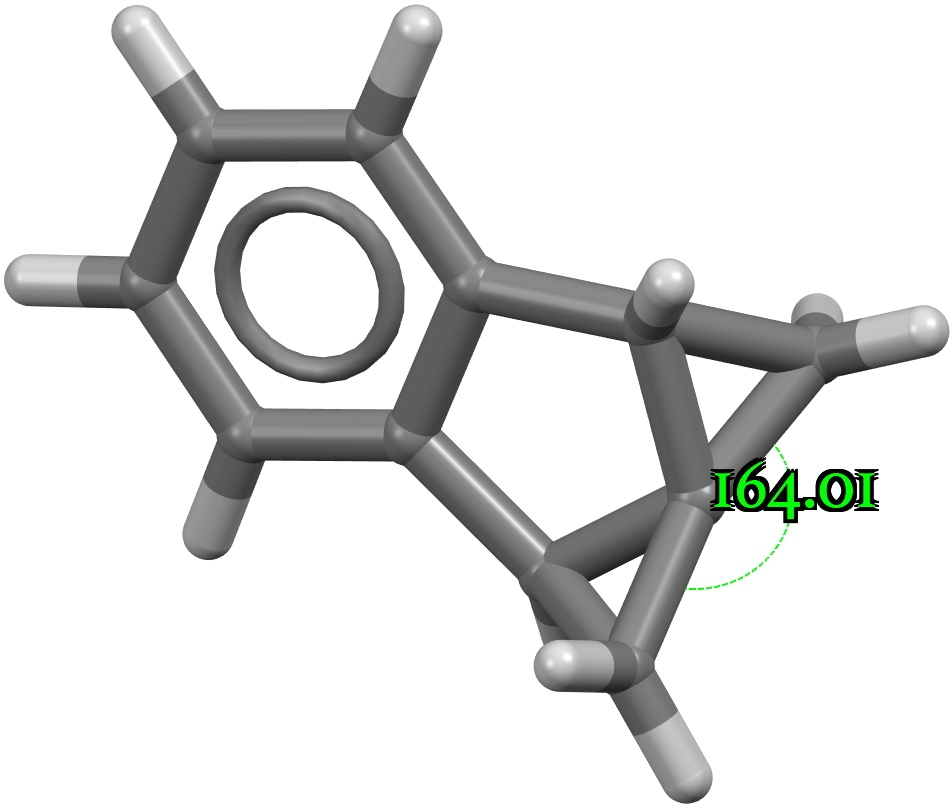
Four-coordinate carbon normally adopts a tetrahedral shape, where the four angles at the carbon are all 109.47°. But how large can that angle get, and can it even get to be 180°? A search of the CSD (crystal structure database) reveals a spiropentane as having the largest such angle, VAJHAP with 164°[cite]10.1021/ja00186a058[/cite] Because crystal structures might have artefacts such as disorder etc, it is always good to check this with a