The π 2 + π 2 cyclodimerisation of cis -butene is the simplest cycloaddition reaction with stereochemical implications. I here give it the same treatment as I did previously for electrocyclic pericyclic reactions. The photochemical reaction is known to give a mixture of two tetramethylcyclobutanes in the ratio of 1.3:1.0, with the all- cis isomer apparently predominating.
Woodward and Hoffmann published their milestone article “Stereochemistry of Electrocyclic Reactions” in 1965. This brought maturity to the electronic theory of organic chemistry, arguably started by the proto-theory of Armstrong some 75 years earlier. Here, I take a modern look at the archetypal carrier of this insight, the ring opening of dimethylcyclobutene.
In the previous post, I wrote about the processes that might be involved in a molecular wheel rotating. A nano car has four wheels, and surely the most amazing thing is how the wheels manage to move in synchrony.
The world’s smallest nano car was recently driven a distance of 6nm along a copper track. When I saw this, I thought it might be interesting to go under the hood and try to explain what makes its engine tick and its fuel work.
The epoxidation of an alkene to give an oxirane is taught in introductory organic chemistry. Formulating an analogous mechanism for such reaction of an alkyne sounds straightforward, but one gradually realises that it requires raiding knowledge from several other areas of (perhaps slightly more advanced) chemistry to achieve a joined up approach to the problem.
Following on from Armstrong’s almost electronic theory of chemistry in 1887-1890, and Beckmann’s radical idea around the same time that molecules undergoing transformations might do so via a reaction mechanism involving unseen intermediates (in his case, a transient enol of a ketone) I here describe how these concepts underwent further evolution in the early 1920s.
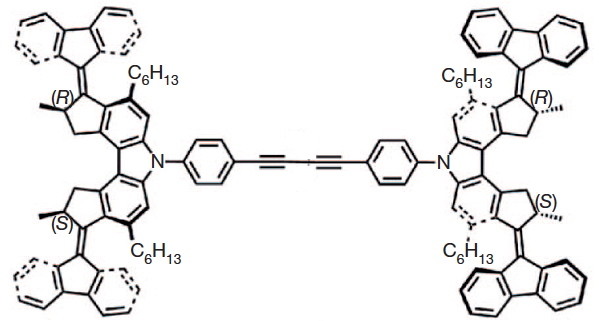
Fascination with nano-objects, molecules which resemble every day devices, is increasing. Thus the world’s smallest car has just been built. The mechanics of such a device can often be understood in terms of chemical concepts taught to most students. So I thought I would have a go at this one!
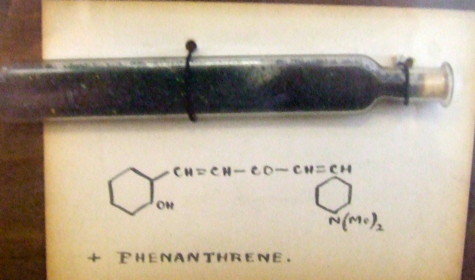
Henry Armstrong studied at the Royal College of Chemistry from 1865-7 and spent his subsequent career as an organic chemist at the Central College of the Imperial college of Science and technology until he retired in 1912.
An attosecond is 10-18s. The chemistry that takes place on this timescale is called electron dynamics. For example, it is the time taken for an electron to traverse the 1s orbit in a hydrogen atom. And chemists are starting to manipulate electrons (and hence chemistry) on this timescale;
How one might go about answering the question: do alkenes promote anomeric effects?
My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down.