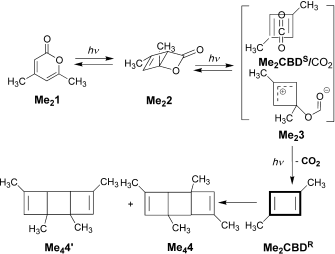
I have mentioned Lewis a number of times in these posts; his suggestion of the shared electron covalent bond still underpins much chemical thinking. Take for example mechanistic speculations on the course of a reaction, a very common indulgence in almost all articles reporting such, and largely based on informed * arrow pushing*. This process is bound to follow the rules of reasonable Lewis structures for any putative intermediates.