Members of the chemical FAIR data community have just met in Orlando (with help from the NSF, the American National Science Foundation) to discuss how such data is progressing in chemistry. There are a lot of themes converging at the moment.
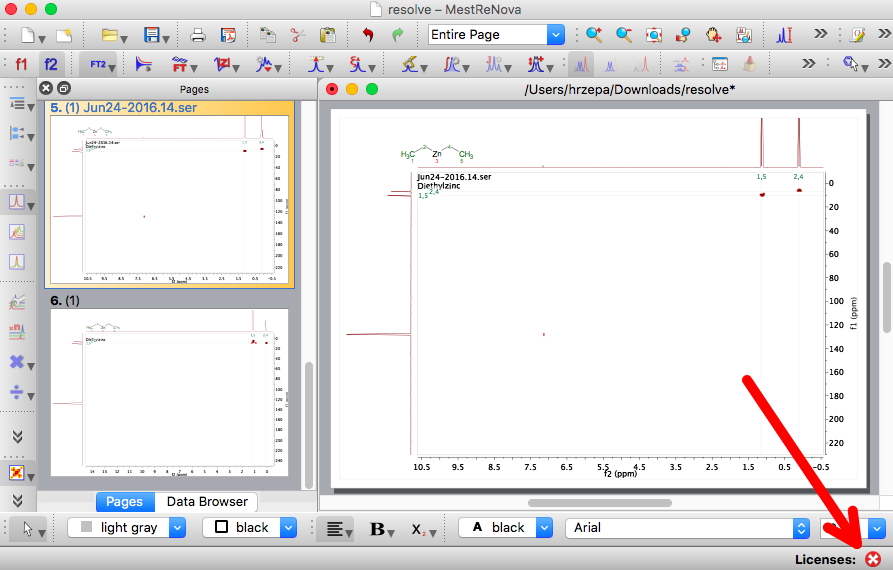
In March, I posted from the ACS meeting in San Diego on the topic of Research data: Managing spectroscopy-NMR, and noted a talk by MestreLab Research on how a tool called Mpublish in the forthcoming release of their NMR analysis software Mestrenova could help. With that release now out, the opportunity arose to test the system.
The upcoming ACS national meeting in San Diego has a CINF (chemical information division) session entitled "Global initiatives in research data management and discovery". I have highlighted here just one slide from my contribution to this session, which addresses the discovery aspect of the session. Data, if you think about it, is rarely discoverable other than by intimate association with a narrative or journal article.
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence?
In 1993-1994, when the Web (synonymous in most minds now with the Internet) was still young, the pace of progress was so rapid that some wag worked out that one “ web-year ” was like a dog-year, worth about 7 years of normal human time. So in this respect, 1994 is now some 133 web-years ago. Long enough for an archaeological excavation.
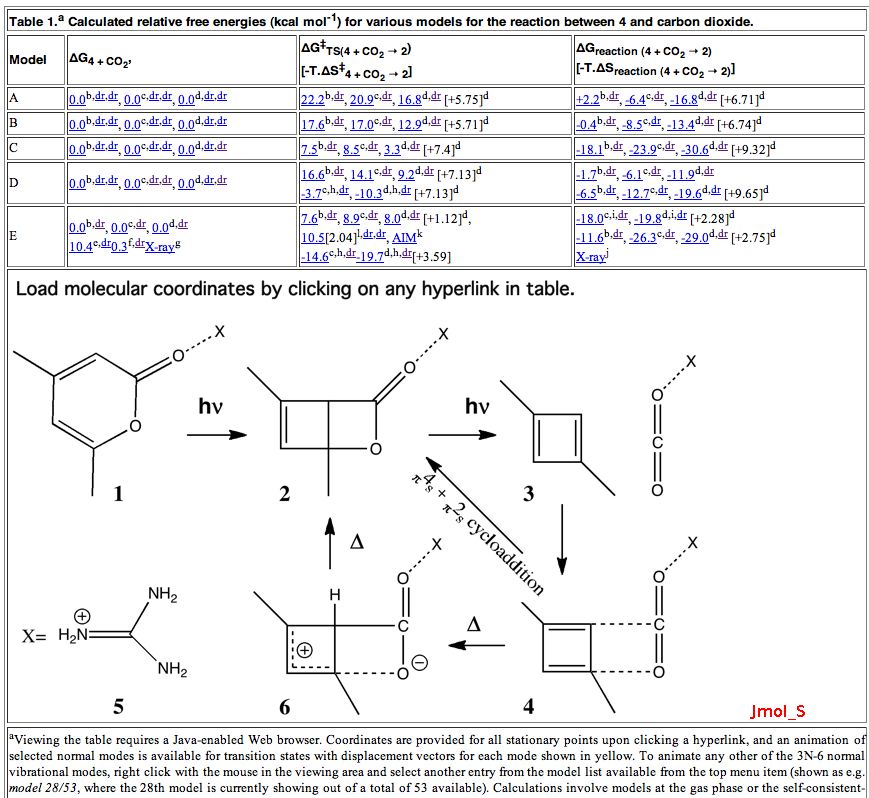
The transient π-complex formed during the “[5,5]” sigmatropic rearrangement of protonated N,O-diphenyl hydroxylamine can be (formally) represented as below, namely the interaction of a six-π-electron aromatic ring (the phenoxide anion 2 ) with a four-π-electron phenyl dication-anion pair 1 . Can one analyse this interaction in terms of aromaticity?
As a personal retrospective of my use of computers (in chemistry), the Macintosh plays a subtle role. 1985: In the previous part, I noted how the Corvus Concept computer introduced a network hard drive (these still being too expensive for any one individual to afford one); the same principle applied to the 1985 Macintosh but now relating to the remarkable introduction of the laser printer.
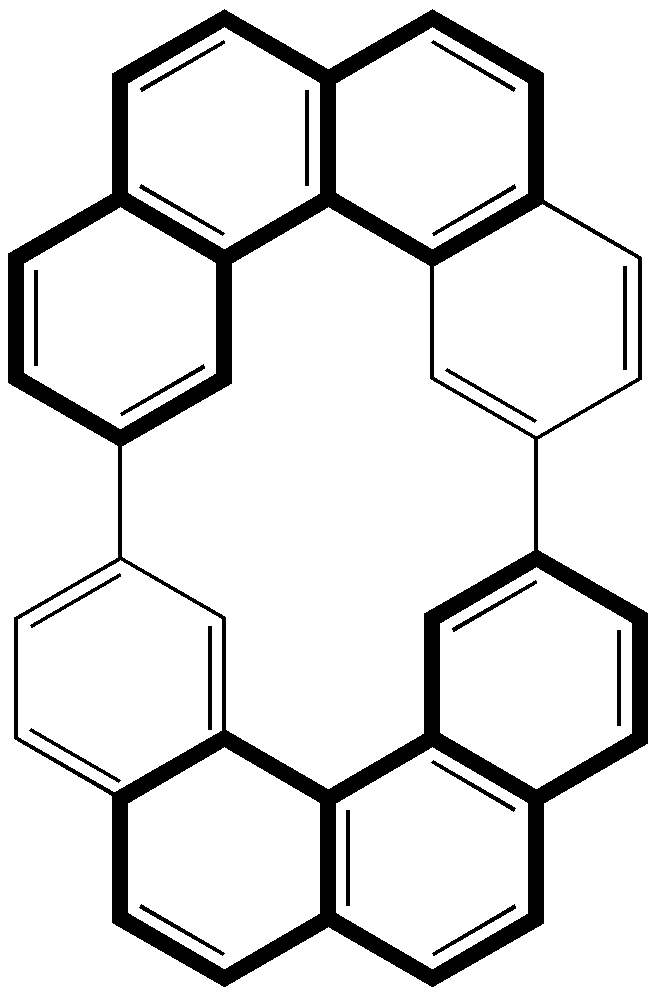
It is not often that an article on the topic of illusion and deception makes it into a chemical journal.
Moving (chemical) data around in a manner which allows its (automated) use in whichever context it finds itself must be a holy grail for all scientists and chemists.