An investigation of the kinetics of the reaction between titanocene pentasulfide and sulfenyl chloride leading to the formation of the S7 allotrope of sulfur was accompanied by supporting DFT calculations which led to the conclusion that of five possible mechanisms for the reaction, the most probable corresponded to a variant of the concerted σ-bond metathesis (Scheme 1, […]
Reinvestigating the reported transition state structure of a concerted triple H-tunneling mechanism.
Substituting a deuterium isotope (2H) for a normal protium hydrogen isotope can slow the rate of a chemical reaction if this atom is involved in the reaction mode.
In the previous post, I was commenting that the transition state for borohydride reduction of a ketone contained some close contacts between the hydrogen of the borohydride and the hydrogen of water.
Part one of this topic was posted more than ten years ago. I clearly forgot about it, so belatedly, here is part 2 – dealing with the stereochemistry of the reduction of tert-butyl-cyclohexanone by borohydride in water.
In the previous post I mooted the possibility that a high energy form of the dimer of nitric oxide 1 might nonetheless be able to be detected using suitable traps (such as hydrogenation or cycloaddition). However, an interesting alternative is that this species could be trapped by nitric oxide itself.
Previously I looked at some of the properties of the mysterious dimer of nitric oxide 1 – not the known weak dimer but a higher energy form with a “triple” N≡N bond.
I started adding citations to my blog posts around 2012 using the Kcite plugin.‡ Rogue Scholar is a service that monitors registered blog sources and adds all sorts of value to the original post, including identifying such citations and creating a list of them.
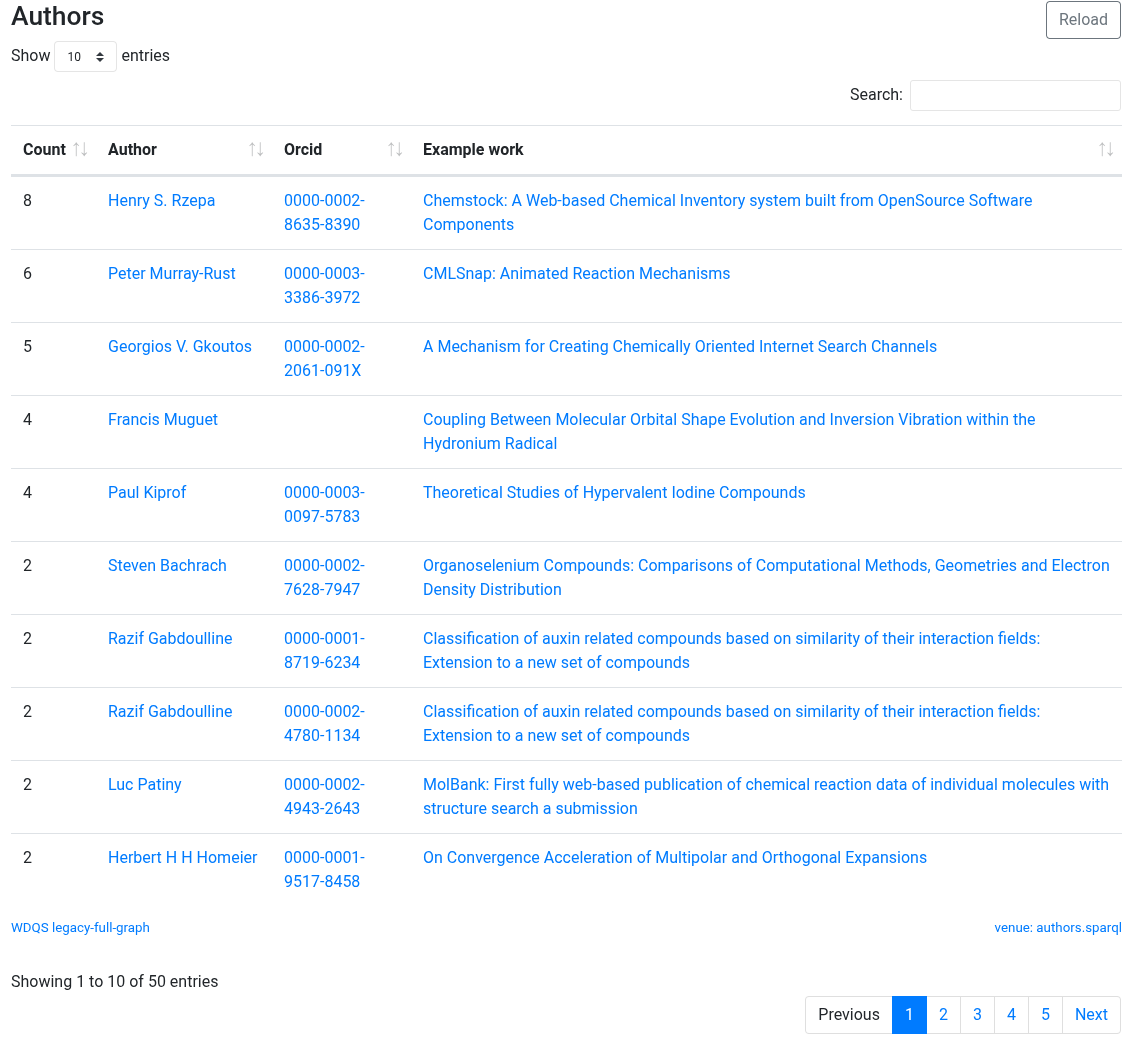
Two years ago, I posted on the topic “Internet Archeology: reviving a 2001 article published in the Internet Journal of Chemistry (IJC)”. The IJC had been founded in 1998, in part at least to “re-invent” the scholarly journal by elevating research data to being a more integrated part of the overall article, rather than as […]
Previously, I pondered about the strange N=N double bond in nitrosobenzene dimer as a follow up to commenting on the curly arrow mechanism of the dimerisation.
In the previous post, I introduced the N=N double bond in nitrosobenzene dimer, arguing that even though it was a formal double bond, its bond dissociation energy made it nonetheless a very weak double bond! This was backed up by a technique known as energy decomposition analysis or EDA.
In an earlier blog, I discussed the curly arrows associated with the known dimerisation of nitrosobenzene, and how the N=N double bond (shown in red below) forms in a single concerted process.