In the preceding post, I looked at a computed mechanism for the hydrolysis of a ketal by water.
The previous post was about an insecticide and made a point that the persistence of both insecticides and herbicides is an important aspect of their environmental properties. Water hydrolysis will degrade them, a typical residency time being in the order of a few days.
Deltamethin is a pyrethroid insecticide for control of malaria which has been used for a little while. Perhaps inevitably, mosquitoes are developing resistance to it. So what could be done about countering this? Well, perhaps surprisingly, form a polymorph! These crystal structure isomers are often highly undesirable;
For obvious reasons, anti-viral molecules are very much in the news at the moment. Thus Derek Lowe highlights Molnupiravir which is shown as a hydroxylamine, the representation originating from the Wikipedia page on the molecule.
I have occasionally covered the topic of colours here, such as those of flowers and minerals, since it is at least possible to illustrate these using photographs or colour charts to illustrate the theme.
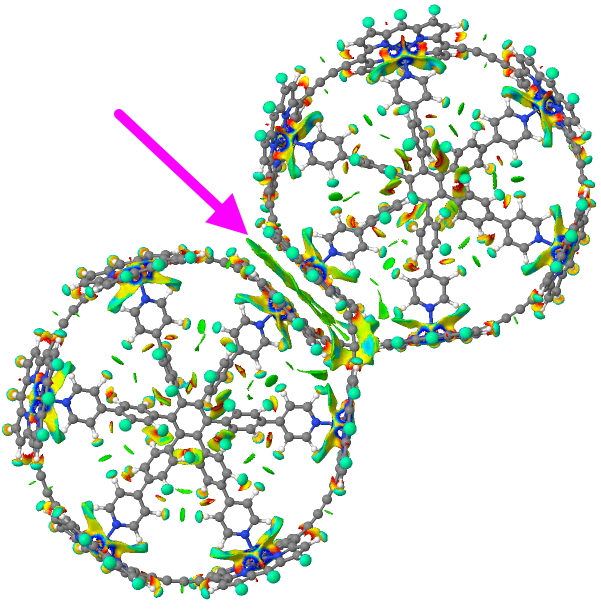
The last post addressed the concept of “steric clashes” in a pericyclic reaction transition state as an extension of the time honoured practice of building molecular models to analyse reaction outcomes. A modern computer generated model might express this in terms of a NCI (non-covalent-interaction) surface.
Another foray into one of the more famous anecdotal chemistry “models”, the analysis of which led directly to the formulation of the WoodWard-Hoffmann (stereochemical) rules for pericyclic reactions.
In a recent post, I told the story of how in the early 1960s, Robert Woodward had encountered an unexpected stereochemical outcome to the reaction of a hexatriene, part of his grand synthesis of vitamin B12.
Last May, I wrote an update to the story sparked by the report of the chemical synthesis of C2. This species has a long history of spectroscopic observation in the gas phase, resulting from its generation at high temperatures.
The quote of the post title comes from R. B. Woodward explaining the genesis of the discovery of what are now known as the Woodward-Hoffmann rules for pericyclic reactions.
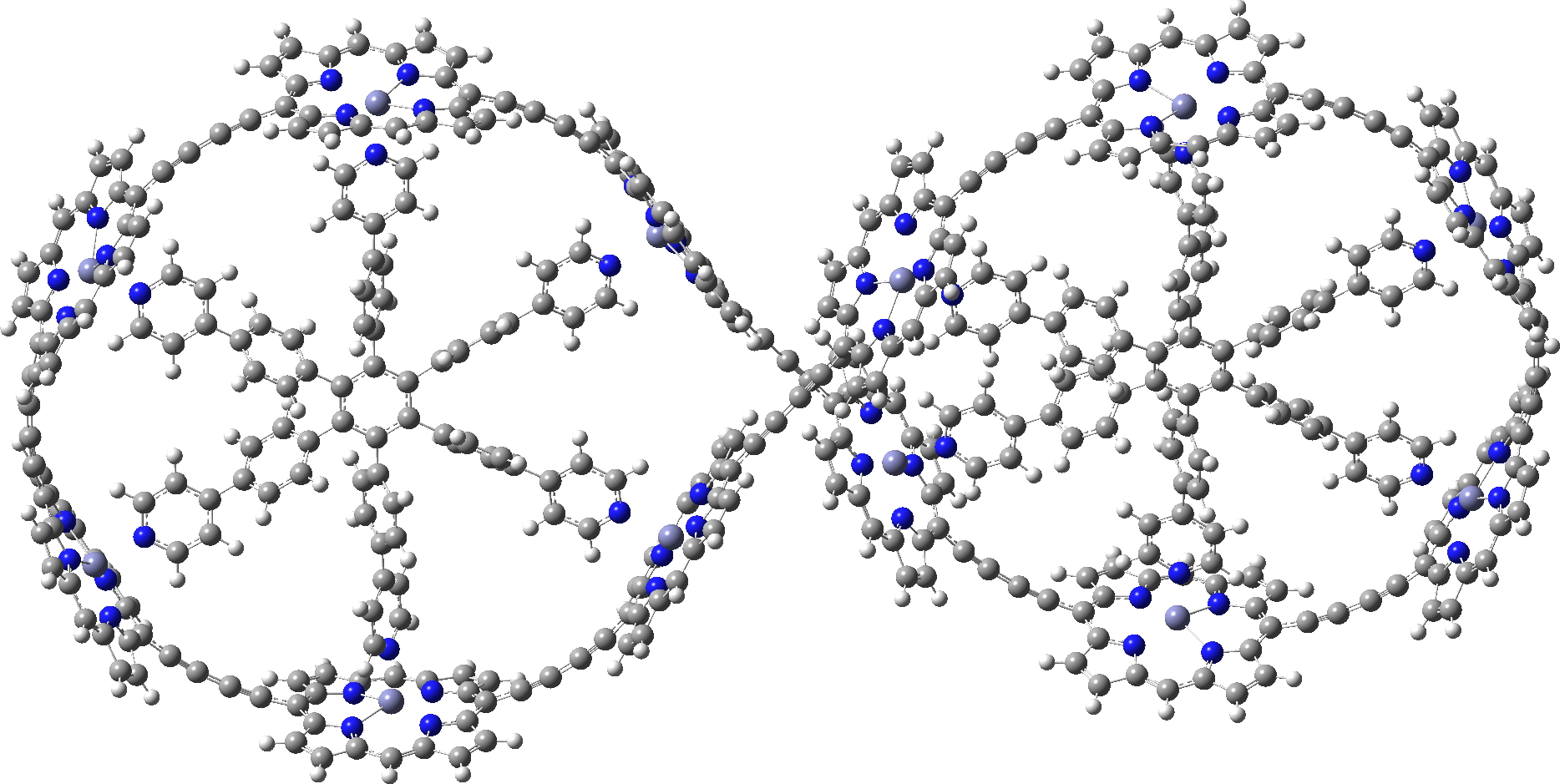
In the previous post, I showed the geometries of three large cyclic porphyrins, as part of an article on exploring the aromaticity of large 4n+2 cyclic rings. One of them had been induced into a “figure-eight” or lemniscular conformation, as shown below.