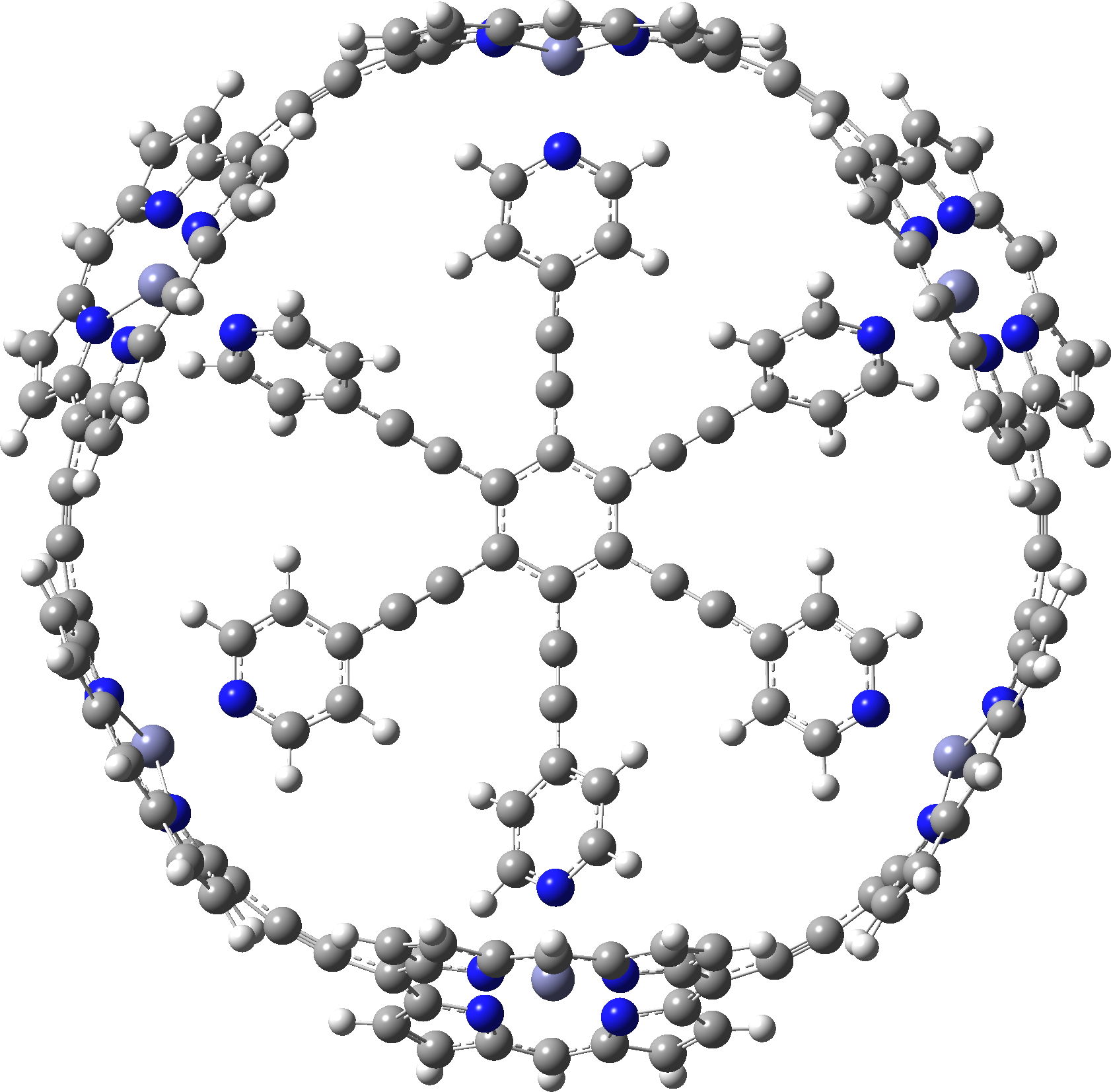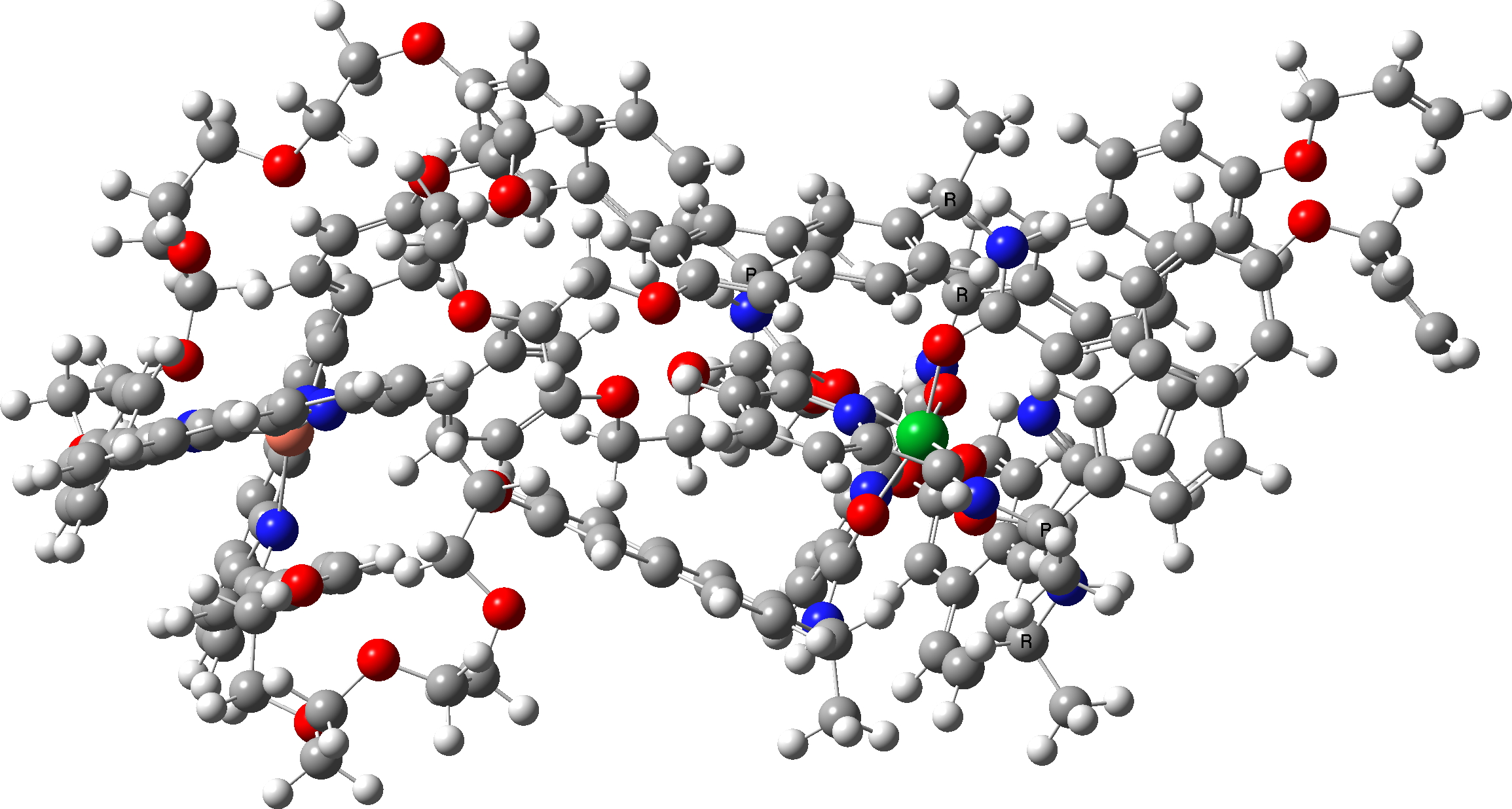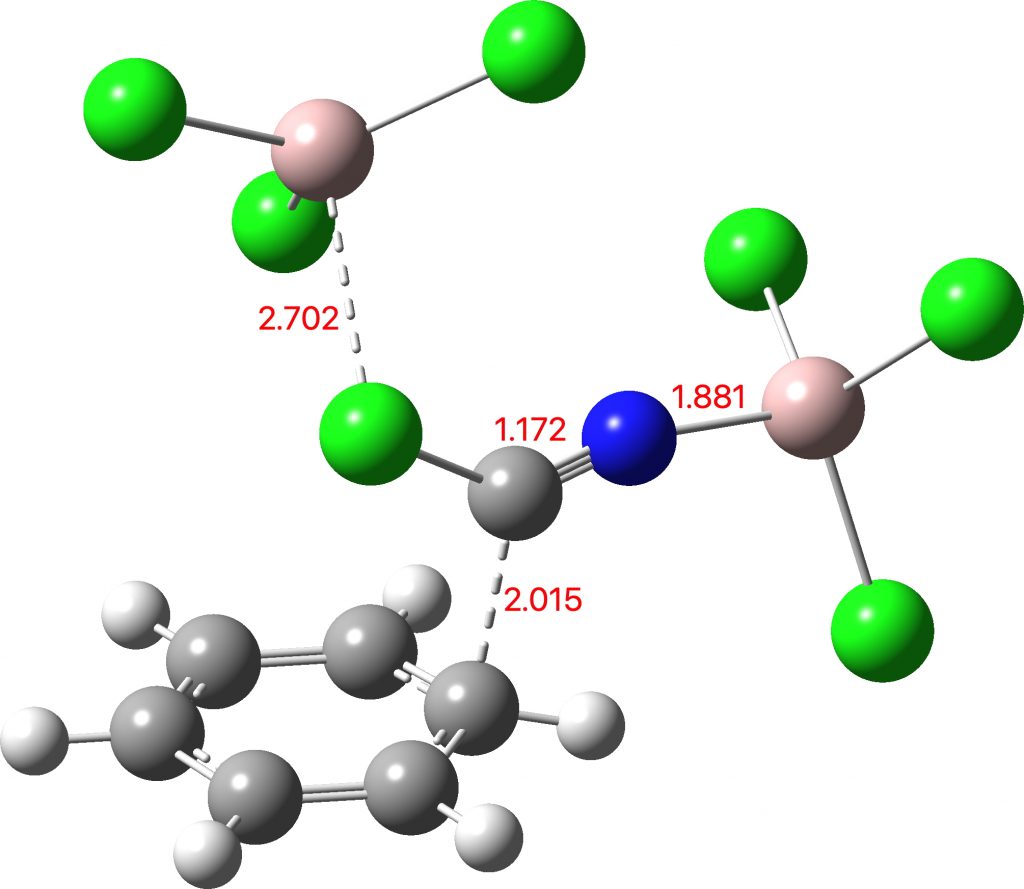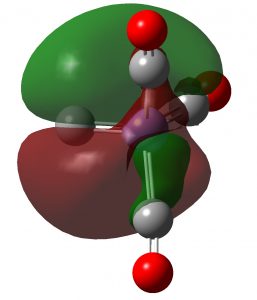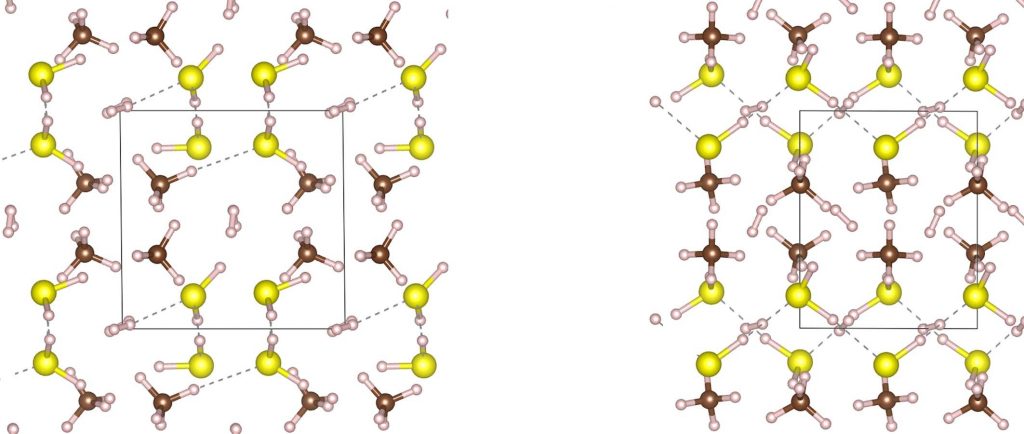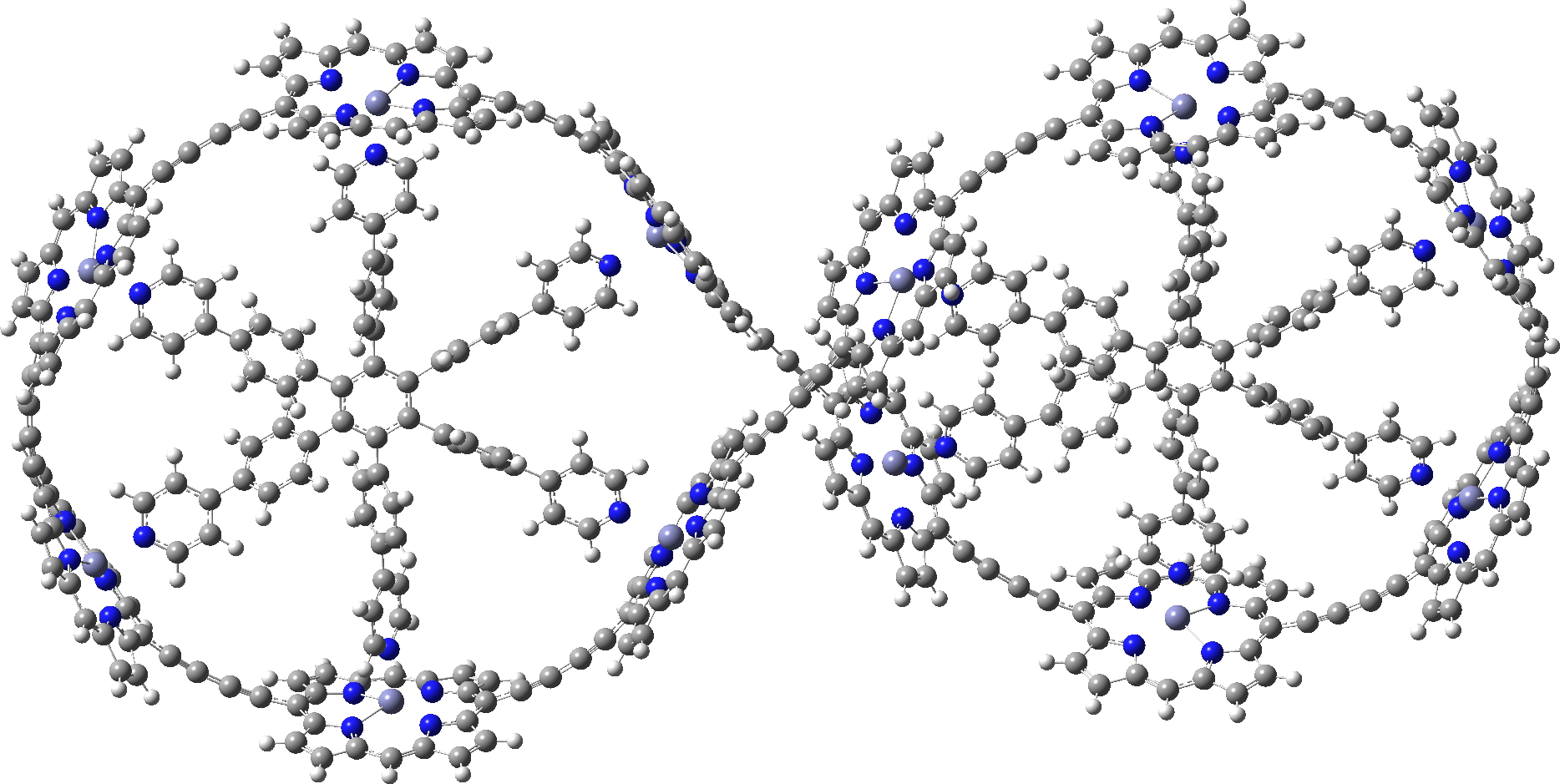
In the previous post, I showed the geometries of three large cyclic porphyrins, as part of an article on exploring the aromaticity of large 4n+2 cyclic rings. One of them had been induced into a “figure-eight” or lemniscular conformation, as shown below.