Here I investigate a recent report of a new generation of polyesters with the intrinsic properties of high crystallinity and chemical recyclability. The latter point is key, since many current plastics cannot be easily recycled to a form which can be used to regenerate the original polymer with high yield.
The folks at DataCite have announced a new research object discovery service which aims to give users a “comprehensive overview of connections between entities in the research landscape”. The portal https://commons.datacite.org acts as the entry point for three basic types of persistent identifiers (PIDs);
The two previous surveys of the potential energy surface for this, it has to be said, rather obscure reaction led to energy barriers that were rather to high to be entirely convincing. So here is a third possibility.
Continuing an exploration of the mechanism of this reaction, an alternative new mechanism was suggested in 1989 (having been first submitted to the journal ten years earlier!). Here the key intermediate proposed is a thiirenium cation (labelled 8 in the article) and labelled Int3 below.
Sometimes a (scientific) thought just pops into one’s mind. Most are probably best not shared with anyone, but since its the summer silly season, I thought I might with this one.
The Willgerodt reaction, discovered in 1887 and shown below, represents a transformation with a once famously obscure mechanism. A major step in the elucidation of that mechanism came using the then new technique of 14C radio-labelling, shortly after the atom bomb projects during WWII made 14CO2 readily available to researchers.
One of the most fascinating and important articles dealing with curly arrows I have seen is that by Klein and Knizia on the topic of C-H bond activations using an iron catalyst.
Metaldehyde is an insecticide used to control slugs.
With the current global lockdown, and students along with everyone else staying at home, I have noticed some old posts of mine are getting more attention than normal. One of these is an analysis I did in 2012 of Robinson’s original curly arrow illustration.
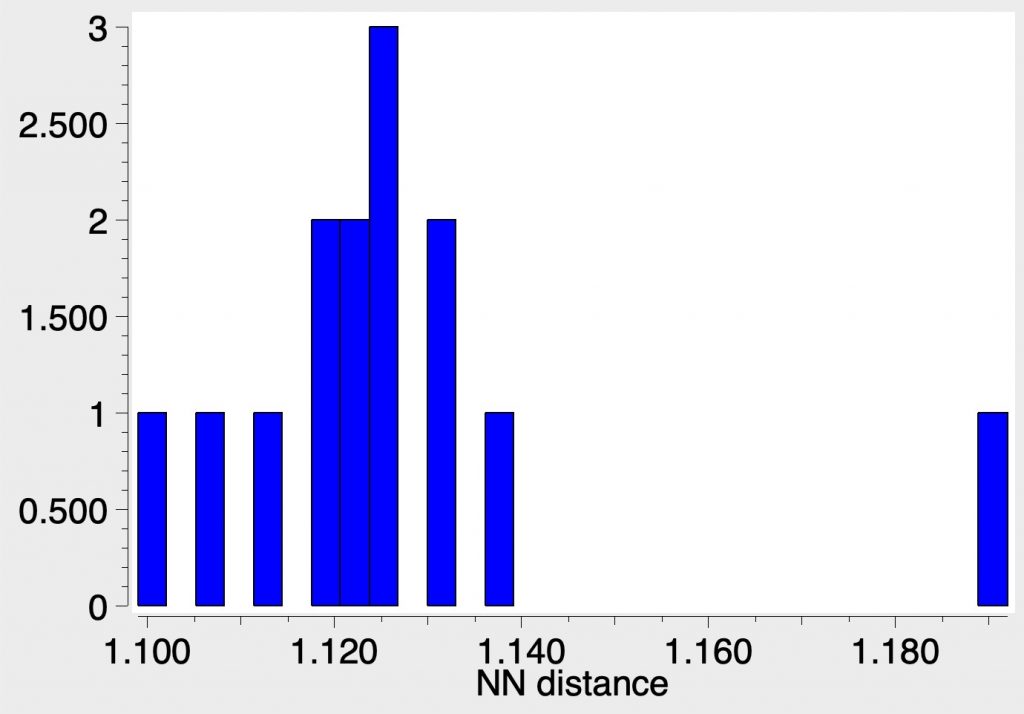
My previous two posts on the topic of strongest bonds have involved mono and diprotonating N2 and using quantum mechanics to predict the effect this has on the N-N bond via its length and vibrational stetching mode. Such species are very unlikely to be easily observed for verification.
I occasionally notice that posts that first appeared here many years ago suddenly attract attention.