Linear free energy relationships (LFER) are associated with the dawn of physical organic chemistry in the late 1930s and its objectives in understanding chemical reactivity as measured by reaction rates and equilibria.
There is emerging interest in cyclic conjugated molecules that happen to have triplet spin states and which might be expected to follow a 4n rule for aromaticity.[cite]10.1002/anie.201705228[/cite] The simplest such system would be the triplet state of cyclobutadiene, for which a non or anti-aromatic singlet state is always found to be lower in energy. Here I explore some crystal structures containing this motif for possible insights.
The traditional structure of the research article has been honed and perfected for over 350 years by its custodians, the publishers of scientific journals. Nowadays, for some journals at least, it might be viewed as much as a profit centre as the perfected mechanism for scientific communication. Here I take a look at the components of such articles to try to envisage its future, with the focus on molecules and chemistry.
Five years back, I speculated about the mechanism of the epoxidation of ethene by a peracid, concluding that kinetic isotope effects provided interesting evidence that this mechanism is highly asynchronous and involves a so-called “hidden intermediate”. Here I revisit this reaction in which a small change is applied to the atoms involved. Below are two representations of the mechanism.
For perhaps ten years now, the future of scientific publishing has been hotly debated. The traditional models are often thought to be badly broken, although convergence to a consensus of what a better model should be is not apparently close. But to my mind, much of this debate seems to miss one important point, how to publish data.
The Royal Society of Chemistry historical group (of which I am a member) organises two or three one day meetings a year. Yesterday the October meeting covered (amongst other themes) the fascinating history of madder and its approximately synthetic equivalent alizarin. Here I add a little to the talk given by Alan Dronsfield on the synthesis of alizarin and the impact this had on the entire industry.
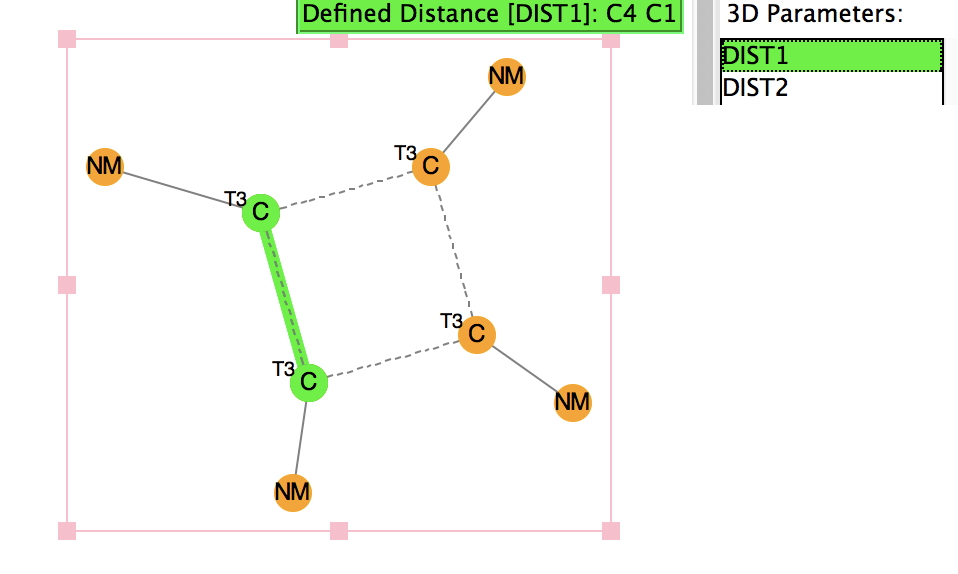
Here is the concluding part of my exploration of a recently published laboratory experiment for undergraduate students.[cite]10.1021/acs.jchemed.7b00566[/cite] I had previously outlined a possible mechanistic route, identifying TS3 (below) as the first transition state in which C-C bond formation creates two chiral centres.
Recently, the 100th anniversary of the birth of the famous chemist Derek Barton was celebrated with a symposium. One of the many wonderful talks presented was by Tobias Ritter and entitled “ Late-stage fluorination for PET imaging ” and this resonated for me. The challenge is how to produce C-F bonds under mild conditions quickly so that 18 F-labelled substrates can be injected for the PET imaging.
I am exploring the fascinating diverse facets of a recently published laboratory experiment for undergraduate students.[cite]10.1021/acs.jchemed.7b00566[/cite] Previously I looked at a possible mechanistic route for the reaction between an enal (a conjugated aldehyde-alkene) and benzyl chloride catalysed by base and a chiral amine, followed by the use of NMR coupling constants to assign relative stereochemistries.
In the previous post, I investigated the mechanism of cyclopropanation of an enal using a benzylic chloride using a quantum chemistry based procedure.
Symbiosis between computation and experiment is increasingly evident in pedagogic journals such as J. Chemical Education . Thus an example of original laboratory experiments[cite]10.1021/ed077p271[/cite],[cite]10.1021/ed078p1266[/cite] that later became twinned with a computational counterpart.[cite]10.1021/ed500398e[/cite] So when I spotted this recent lab experiment[cite]10.1021/acs.jchemed.7b00566[/cite] I felt another twinning
