Members of the chemical FAIR data community have just met in Orlando (with help from the NSF, the American National Science Foundation) to discuss how such data is progressing in chemistry. There are a lot of themes converging at the moment.
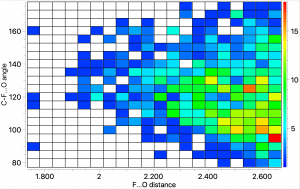
There is a predilection amongst chemists for collecting records; one common theme is the length of particular bonds, either the shortest or the longest.
Students learning organic chemistry are often asked in examinations and tutorials to devise the mechanisms (as represented by curly arrows) for the core corpus of important reactions, with the purpose of learning skills that allow them to go on to improvise mechanisms for new reactions.
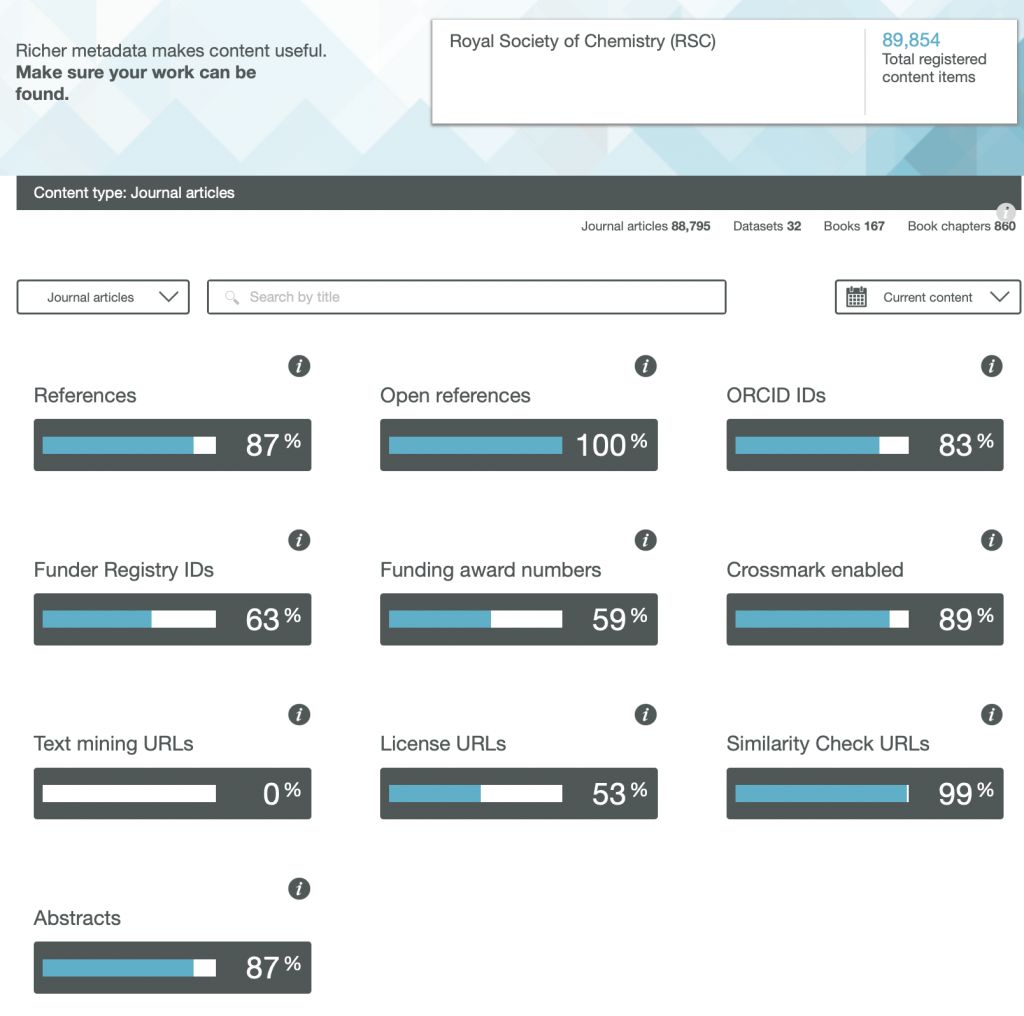
The title of this post comes from the site www.crossref.org/members/prep/ Here you can explore how your favourite publisher of scientific articles exposes metadata for their journal.

The Book of Kells is a spectacularly illuminated gospel manuscript dating from around 800AD and held in Trinity College library in Dublin. Some idea of the colours achieved can be seen below.
Linear free energy relationships (LFER) are associated with the dawn of physical organic chemistry in the late 1930s and its objectives in understanding chemical reactivity as measured by reaction rates and equilibria.
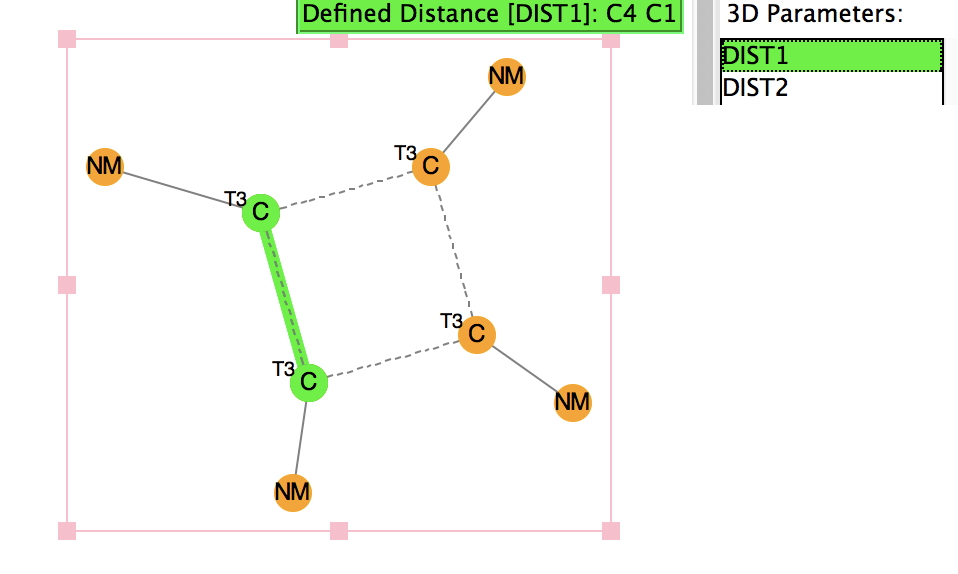
There is emerging interest in cyclic conjugated molecules that happen to have triplet spin states and which might be expected to follow a 4n rule for aromaticity. The simplest such system would be the triplet state of cyclobutadiene, for which a non or anti-aromatic singlet state is always found to be lower in energy.
The traditional structure of the research article has been honed and perfected for over 350 years by its custodians, the publishers of scientific journals. Nowadays, for some journals at least, it might be viewed as much as a profit centre as the perfected mechanism for scientific communication.
Five years back, I speculated about the mechanism of the epoxidation of ethene by a peracid, concluding that kinetic isotope effects provided interesting evidence that this mechanism is highly asynchronous and involves a so-called “hidden intermediate”. Here I revisit this reaction in which a small change is applied to the atoms involved.
For perhaps ten years now, the future of scientific publishing has been hotly debated. The traditional models are often thought to be badly broken, although convergence to a consensus of what a better model should be is not apparently close.
The Royal Society of Chemistry historical group (of which I am a member) organises two or three one day meetings a year. Yesterday the October meeting covered (amongst other themes) the fascinating history of madder and its approximately synthetic equivalent alizarin.