At the ACS conference, I have attended many talks these last four days, but one made some “connections” which intrigued me. I tell its story (or a part of it) here. But to start, try the following experiment. Find a Word document of .docx type on your hard drive Remove the .docx suffix and replace it with a .zip suffix. Expand as if it is an archive (it is!). A folder is created and this itself contains four further folders.
The upcoming ACS national meeting in San Diego has a CINF (chemical information division) session entitled "Global initiatives in research data management and discovery". I have highlighted here just one slide from my contribution to this session, which addresses the discovery aspect of the session. Data, if you think about it, is rarely discoverable other than by intimate association with a narrative or journal article.
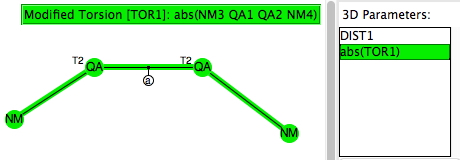
The upcoming ACS national meeting in San Diego has a CHED (chemical education division) session entitled Implementing Discovery-Based Research Experiences in Undergraduate Chemistry Courses. I had previously explored what I called extreme gauche effects in the molecule F-S-S-F. Here I take this a bit further to see what else can be discovered about molecules containing bonds between group 16 elements (QA= O, S, Se, Te).
At the precise moment I write this, there is information about 108,230,950 organic and inorganic chemical substances from the World's disclosed chemistry.

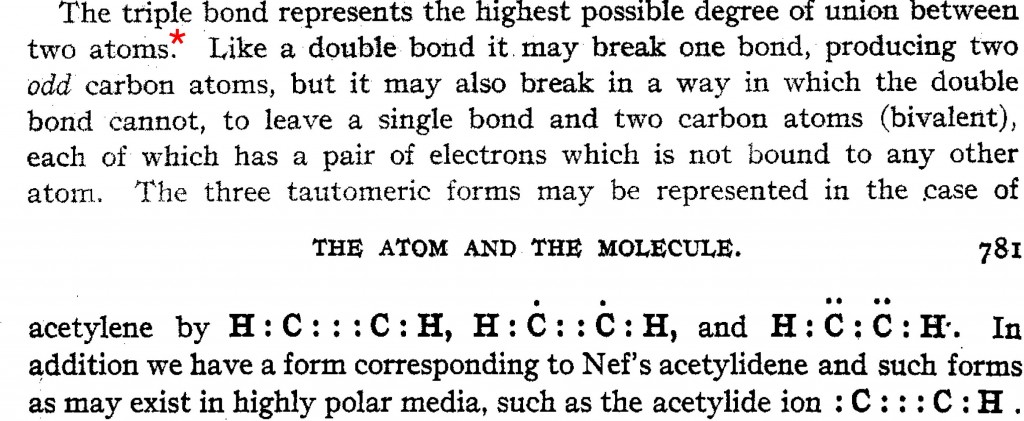
Hypervalency is defined as a molecule that contains one or more main group elements formally bearing more than eight electrons in their valence shell. One example of a molecule so characterised was CLi 6 [cite]10.1038/355432a0[/cite] where the description "“ carbon can expand its octet of electrons to form this relatively stable molecule ” was used.
The phenomenon of bond stretch isomerism , two isomers of a compound differing predominantly in just one bond length, is one of those chemical concepts that wax and occasionally wane.[cite]10.1016/S1631-0748(02)01380-2[/cite] Here I explore such isomerism for the elements Ge, Sn and Pb. In one earlier post, I noted a form of bond stretch isomerism that can arise from a Jahn-Teller distortion ending in two different geometries
The geometry of cyclo-octatetraenes differs fundamentally from the lower homologue benzene in exhibiting slow (nuclear) valence bond isomerism rather than rapid (electronic) bond-equalising resonance.
I attended the first (of a proposed five) workshops organised by LEARN (an EU-funded project that aims to .. .Raise awareness in research data management (RDM) issues & research policy ) on Friday. Here I give some quick bullet points relating to things that caught my attention and or interest. The program (and Twitter feed) can be found at https://learnrdm.wordpress.com where other's comments can also be seen.
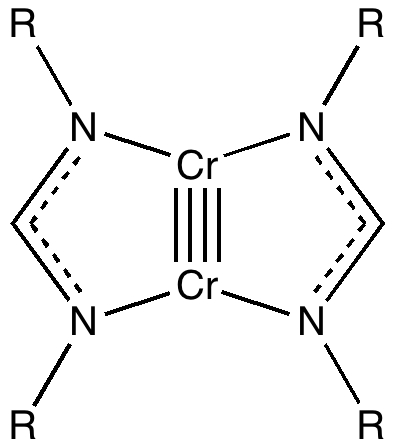
Six years ago, I posted on the nature of a then recently reported[cite]10.1002/anie.200803859[/cite] Cr-Cr quintuple bond. The topic resurfaced as part of the discussion on a more recent post on NSF 3 , and a sub-topic on the nature of the higher order bonding in C 2 . The comment made a connection between that discussion and the Cr-Cr bond alluded to above.
The original strategic objective of my PhD researches in 1972-74 was to explore how primary kinetic hydrogen isotope effects might be influenced by the underlying structures of the transition states involved. Earlier posts dealt with how one can construct quantum-chemical models of these transition states that fit the known properties of the reactions.
The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF 3 has proved perennially popular. So here is a follow-up on another little molecue, F 3 SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis. This is as follows: Six valence electrons on the central S atom.