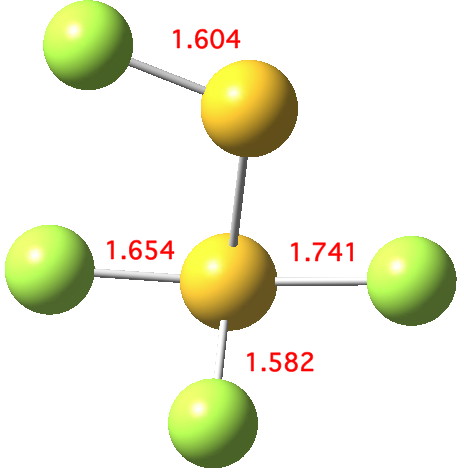
Andy Extance at the Chemistry World blog has picked up on a fascinating article on the dimer of SF2. This molecule has three F atoms on one S, and only one on the other; FSSF3. But all four S-F bonds are of different length.

Andy Extance at the Chemistry World blog has picked up on a fascinating article on the dimer of SF2. This molecule has three F atoms on one S, and only one on the other; FSSF3. But all four S-F bonds are of different length.
According to Herges, the mechanism of single-step (concerted) reactions can be divided into three basic types; linear (e.g. substitution, elimination etc), pericyclic (e.g. Diels Alder) and a third much rarer, and hence very often overlooked type that was named coarctate.
The concept of a “hidden intermediate” in a reaction pathway has been promoted by Dieter Cremer and much invoked on this blog. When I used this term in a recent article of ours, a referee tried to object, saying it was not in common use in chemistry. The term clearly has an image problem.

We have been experimenting with full-colour 3D printing of molecular objects. I thought I might here share some of our observations.
The ultimate reduction in size for an engineer is to a single molecule. It’s been done for a car; now it has been reported for the pixel (picture-element).
The Amsterdam manifesto espouses the principles of citable open data.
Valence shell electron pair repulsion theory is a simple way of rationalising the shapes of many compounds in which a main group element is surrounded by ligands. ClF3 is a good illustration of this theory.
This potential example of a molecule on the edge of chaos was suggested to me by a student (thanks Stephen!), originating from an inorganic tutorial. It represents a class of Mo-complex ligated by two dithiocarbamate ligands and two aryl nitrene ligands (Ar-N:).
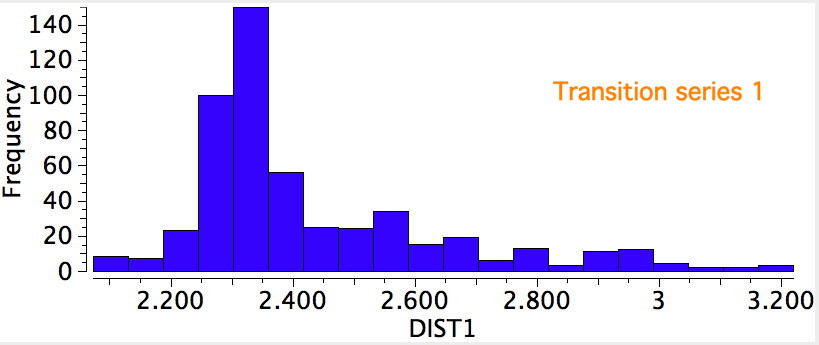
I noted previously that some 8-ring cyclic compounds could exist in either a planar-aromatic or a non-planar-non-aromatic mode, the mode being determined by apparently quite small changes in a ring substituent. Hunting for other examples of such chemistry on the edge, I did a search of the Cambridge crystal database for metal sulfides.
The butterfly effect summarises how a small change to a system may result in very large and often unpredictable (chaotic) consequences. If the system is merely on the edge of chaos, the consequences are predictable, but nevertheless finely poised between e.g. two possible outcomes. Here I ask how a molecule might manifest such behaviour.
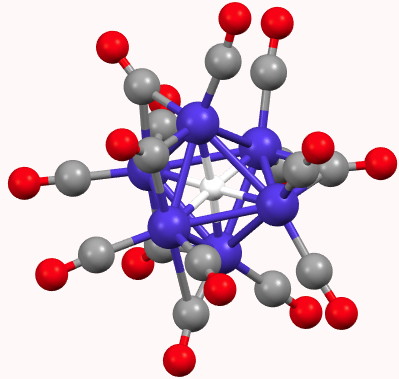
A feature of a blog which is quite different from a journal article is how rapidly a topic might evolve. Thus I started a few days ago with the theme of dicarbon (C2), identifying a metal carbide that showed C2 as a ligand, but which also entrapped a single carbon in hexa-coordinated mode.