Most of the chemical structure diagrams in this blog originate from Chemdraw, which seems to have been around since the dawn of personal computers! I have tended to use this program to produce JPG bitmaps for the blog, writing them out in 4x magnification, so that they can be scaled down for display whilst retaining some measure of higher resolution if needed for other purposes.
I have for perhaps the last 25 years been urging publishers to recognise how science publishing could and should change. My latest thoughts are published in an article entitled “ The past, present and future of Scientific discourse ” (DOI: 10.1186/1758-2946-3-46). Here I take two articles, one published 58 years ago and one published last year, and attempt to reinvent some aspects.
Bonds are a good example of something all chemists think they can recognise when they see them. But they are also remarkably dependent on context. We are running a molecular modelling course at the moment, and I found myself explaining to someone how very context-sensitive they can be. I thought it might be useful to collect my thoughts here.
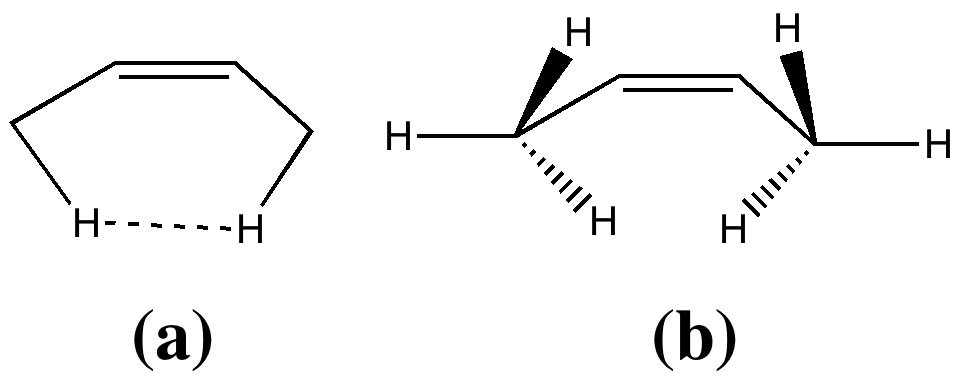
In two previous posts, I have looked at why cis -butene adopts conformation (a) rather than (b). I suggested it boiled down to electronic interactions between the methyl groups and the central alkene resulting in the formation of a H…H “ topological ” bond, rather than attraction between the H…H region to form a weak chemical “ bond ”. Here I take a look at what happens when that central C=C bond is gradually removed.
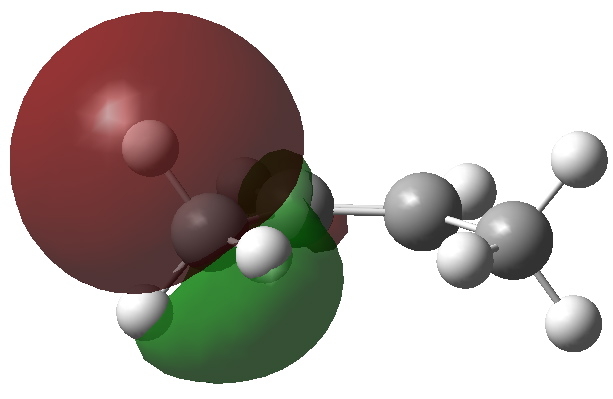
I wrote earlier about the strangely close contact between two hydrogen atoms in cis -butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union?
Steve Jobs death on October 5th 2011 was followed by a remarkable number of tributes and reflections on the impact the company he founded has had on the world. Many of these tributes summarise the effect as a visionary disruption . Here I describe from my own perspective some of the disruptions to chemistry I experienced (for another commentary, see here). Chemical diagram, circa 1983.

The properties of electrons are studied by both chemists and physicists. At the boundaries of these two disciplines, sometimes interesting differences in interpretation emerge. One of the most controversial is that due to Bader (for a recent review, see DOI: 10.1021/jp102748b) a physicist who brought the mathematical rigor of electronic topology to bear upon molecules.
A Matryoshka doll is better known as a Russian nesting doll. They can have up to eight layers. Molecules can only emulate two layers, although see here for a good candidate for making a three-layered example (the inside layer is C 60 , which itself might encapsulate a small molecule. See also DOI: 10.1021/ja991747w). These molecular dolls can be created out of quite simple molecules.

In his famous lecture in 1959, C. P. Snow wrote about the breakdown in communications between the “two cultures” of modern society — the sciences and the humanities (arts). That was then. This is now, and the occasion of my visit to a spectacular “city of arts and sciences complex” in Europe. An un-missable exhibit representing science and life was the 15m high model of DNA.
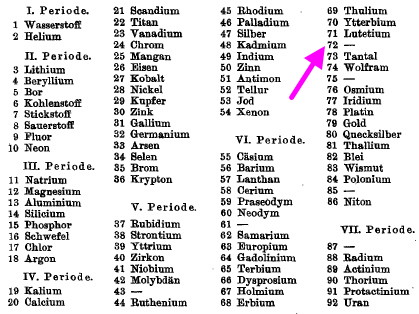
In 1923, Coster and von Hevesy[cite]10.1038/111079a0[/cite] claimed discovery of the element Hafnium , atomic number 72 (latin Hafnia, meaning Copenhagen, where the authors worked) on the basis of six lines in its X-ray spectrum. The debate had long raged as to whether (undiscovered) element 72 belonged to the rare-earth group 3 of the periodic table below yttrium, or whether it should be placed
