In an earlier post on this topic,‡ I described how the curly-arrows describing the mechanism of a nucleophilic addition at a carbonyl group choreograph in two distinct ways, as seen in red or blue below.
Some 13 years ago, I speculated about the longevity of the type of science communication then (and still now) represented by Blogs. I noted one new project called ArchivePress that was looking into providing solutions equivalent to what scientific journals have done for some 350 years of science communication.
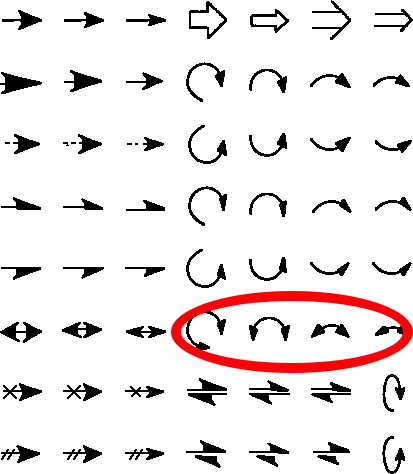
The schematic representation of a chemical reaction mechanism is often drawn using a palette of arrows connecting or annotating the various molecular structures involved. These can be selected from a chemical arrows palette, taken for this purpose from the commonly used structure drawing program Chemdraw.
The Swern oxidation is a class of “activated” dimethyl sulfoxide (DMSO) reaction in which the active species is a chlorodimethylsulfonium chloride salt.

Respiratory pigments are metalloproteins that transport O2, the best known being the bright red/crimson coloured hemoglobin in human blood. The colour derives from Fe2+ at the core of a tetraporphyrin ring.
I have variously talked about persistent identifiers on this blog. These largely take the form of DOIs (Digital object identifiers), and here they relate to either journal articles or datasets associated with either the article or the blog post or both.
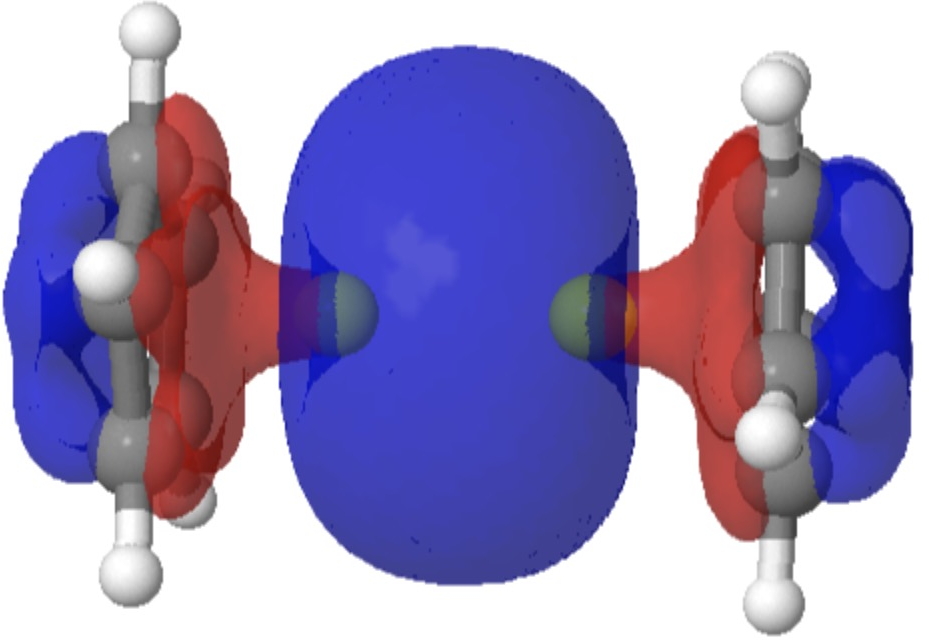
Sometimes, the properties of a molecule are predicted long before it is synthesised. One such is diberyllocene. I first encountered a related molecule, beryllocene itself, many moons ago. This was unusual because unlike the original metallocenes, the metal atom was not symmetrically disposed between the two cyclopentadienyl faces.
This is a venerable organic reaction, which curiously I have not previously covered here. First described in 1859, its nature was only properly elucidated in 1873.
This editorial from Nature is a timely reminder of the importance of data.
Some time ago in 2010, I showed a chemical problem I used to set during university entrance interviews. It was all about pattern recognition and how one can develop a hypothesis based on this.
One of the important aspects of chemical reaction mechanisms is the order in which things happen. More specifically, the order in which bonds make or break when there are more than two involved in undertaking a reaction.