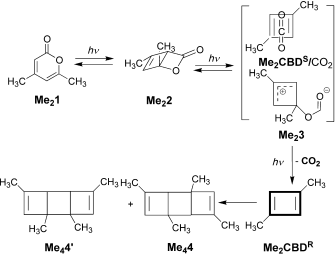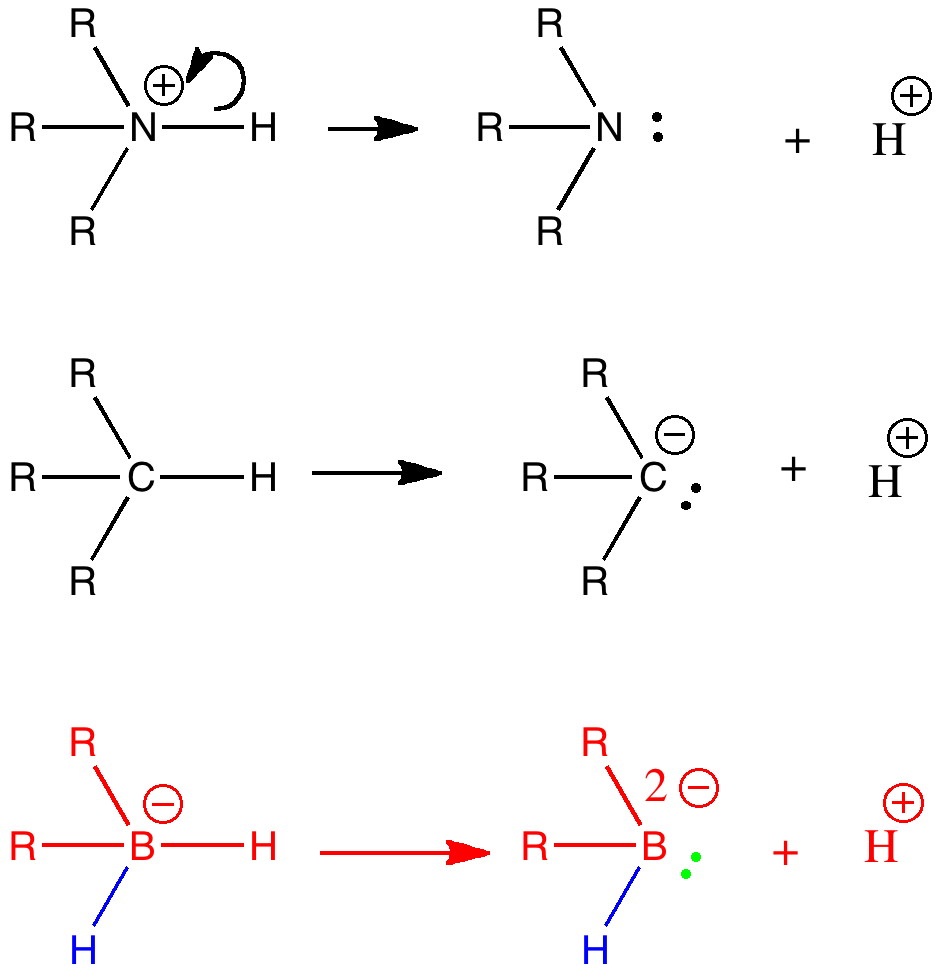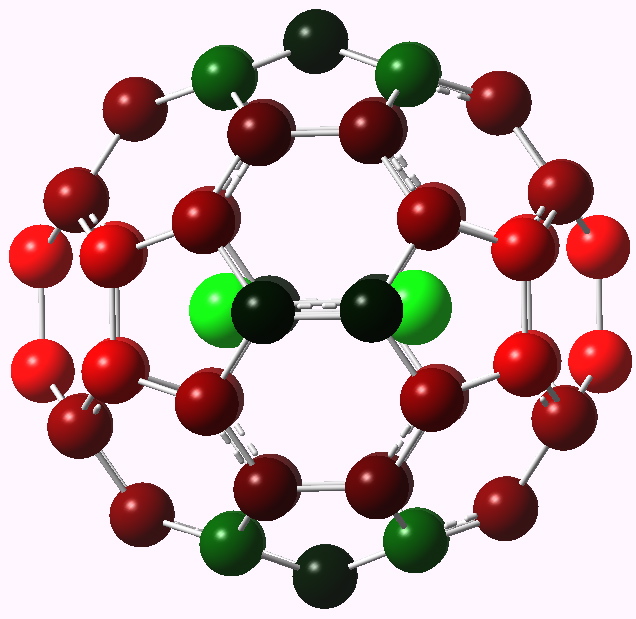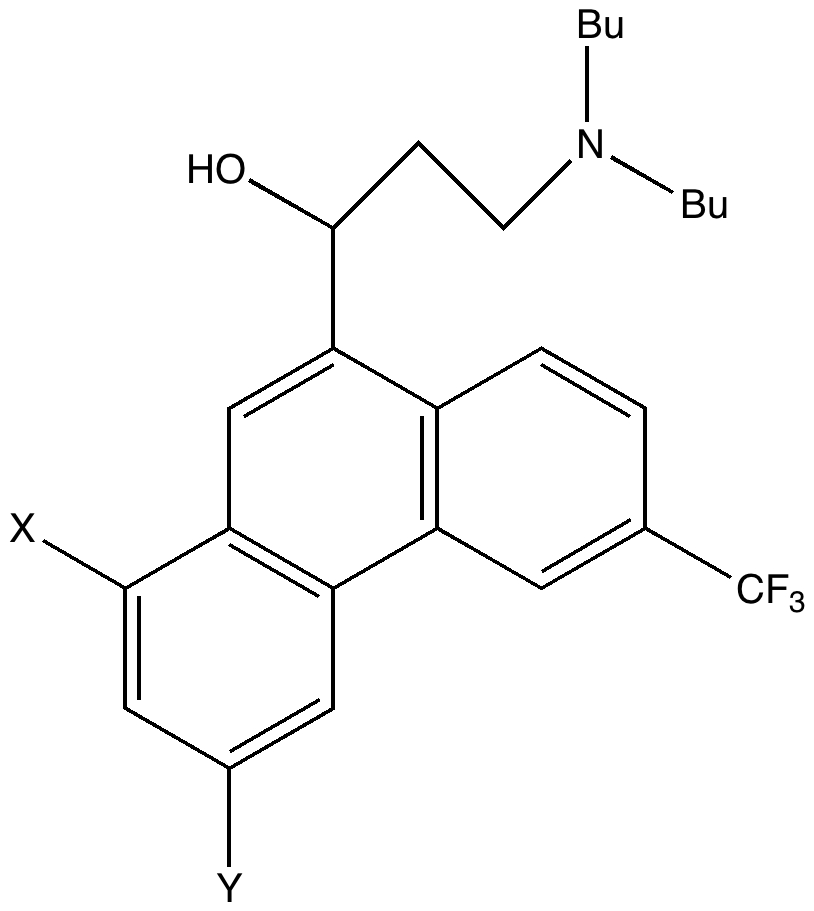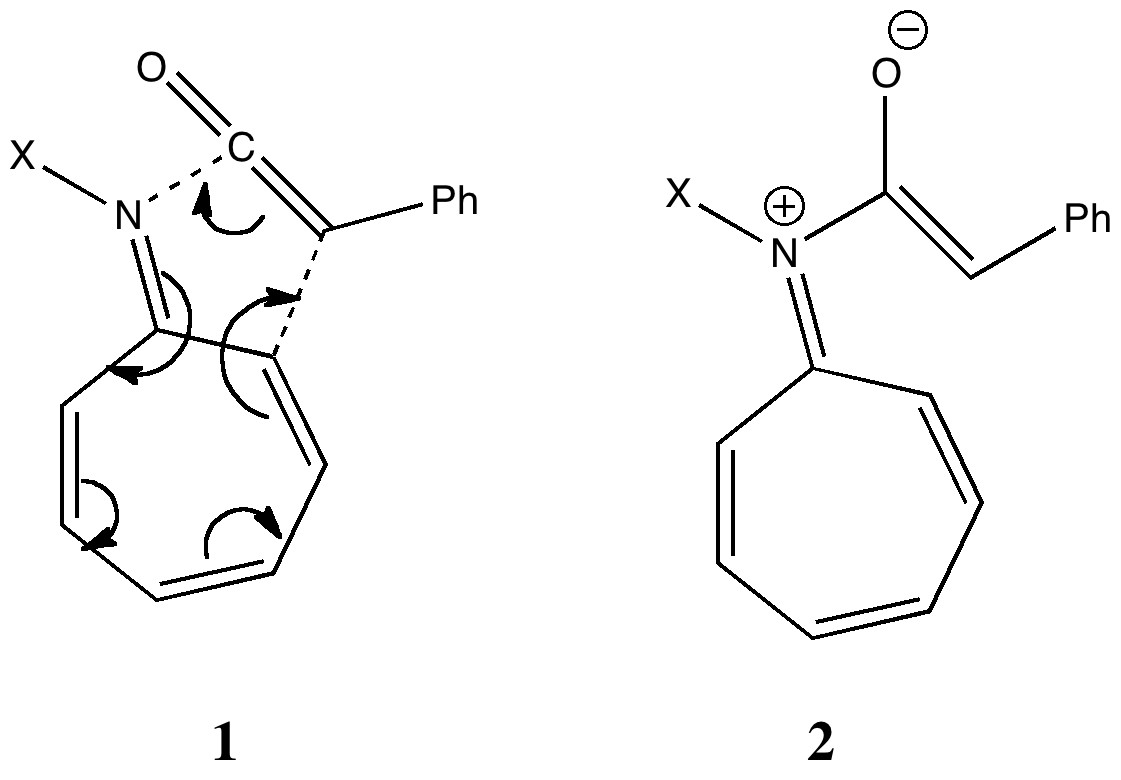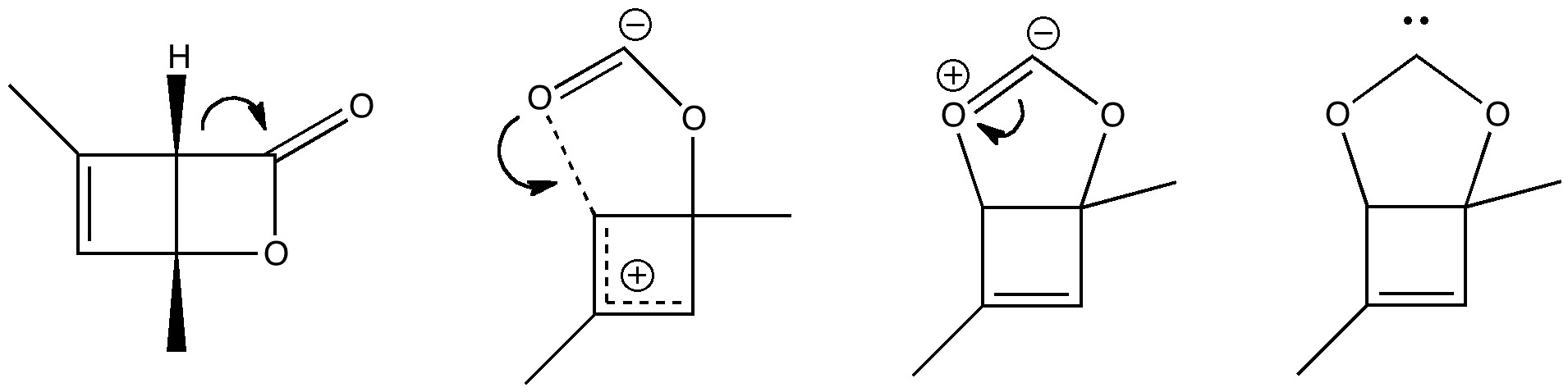
The (hopefully tongue-in-cheek) title Mindless chemistry was given to an article reporting an automated stochastic search procedure for locating all possible minima with a given composition using high-level quantum mechanical calculations. “Many new structures, often with nonintuitive geometries, were found”. Well, another approach is to follow unexpected hunches.