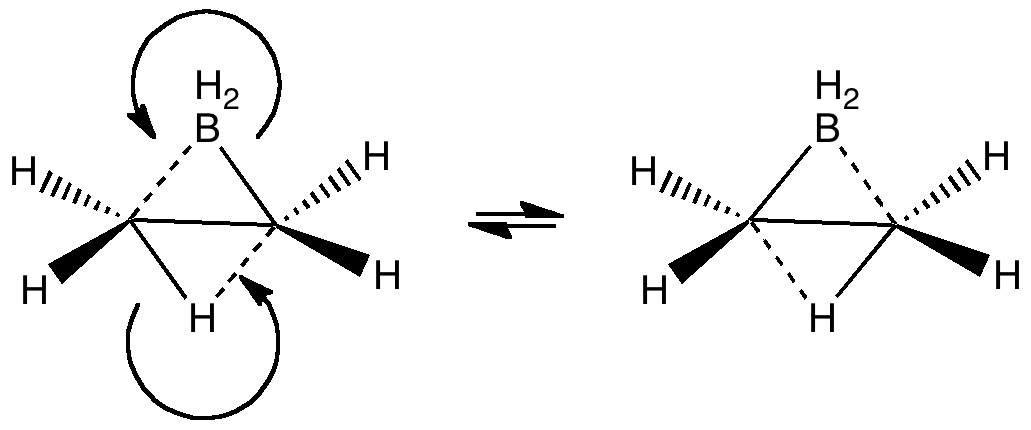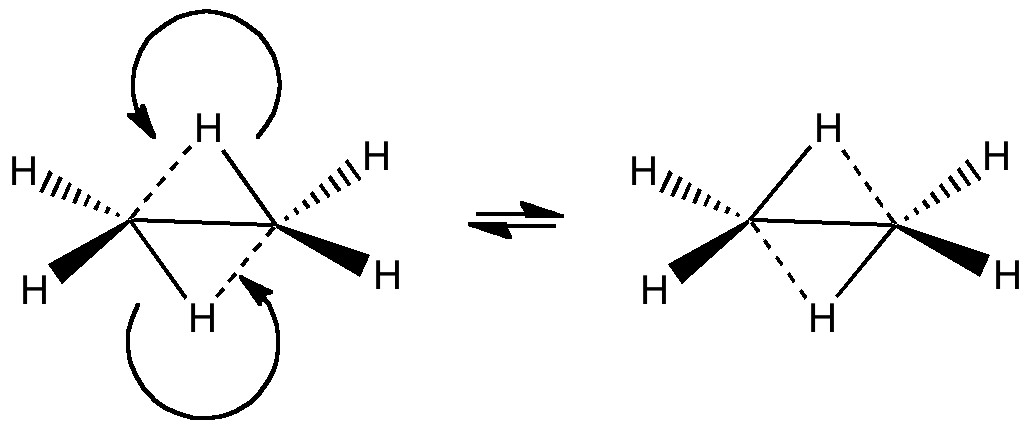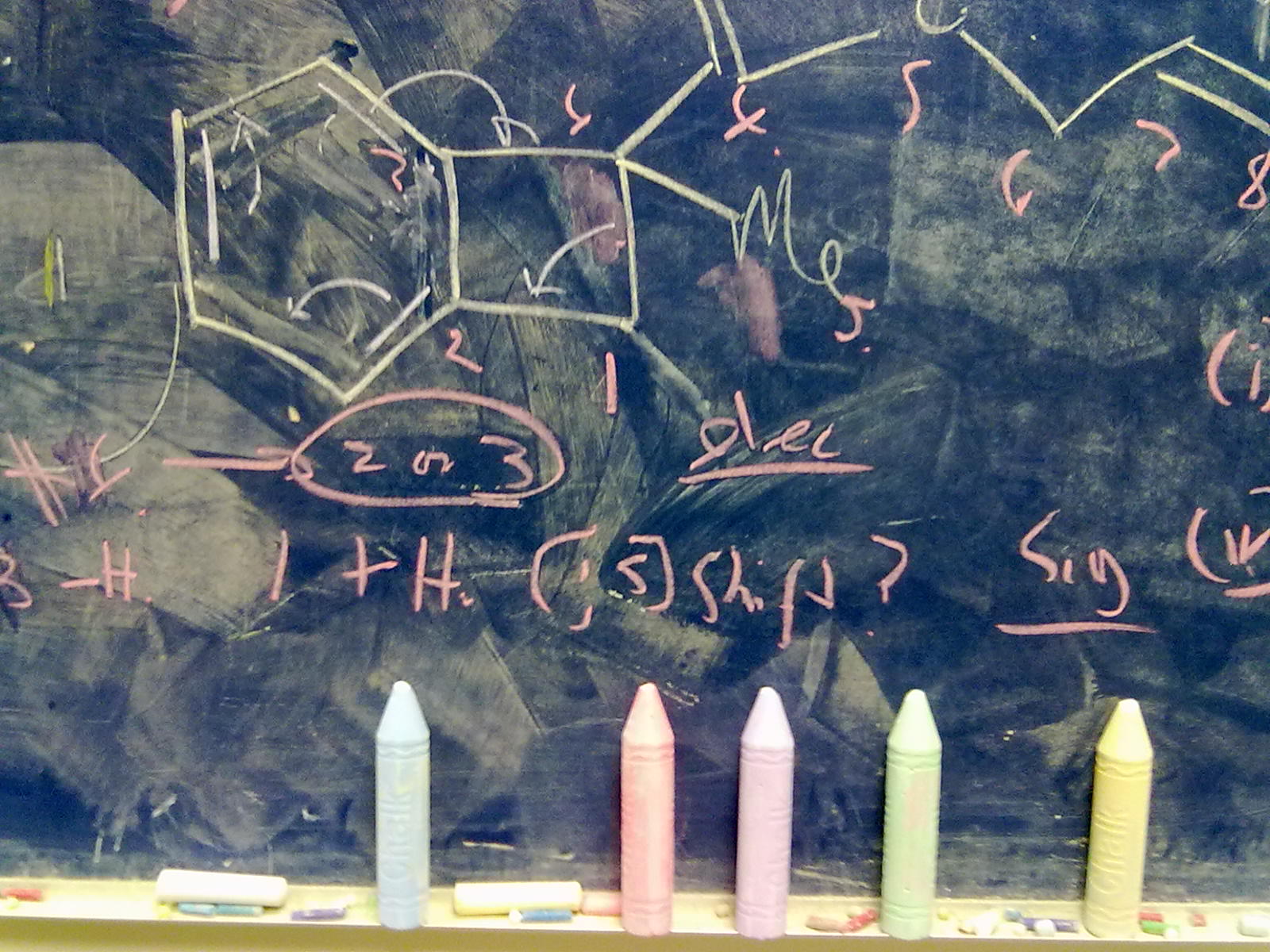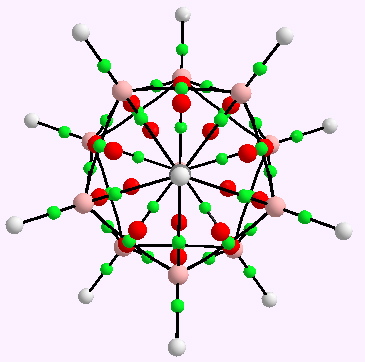
A little while ago, I speculated (blogs are good for that sort of thing) about hexavalent carbon, and noted how one often needs to make (retrospectively) obvious connections between two different areas of chemistry. That post has attracted a number of comments in the two years its been up, along the lines: what about carboranes?