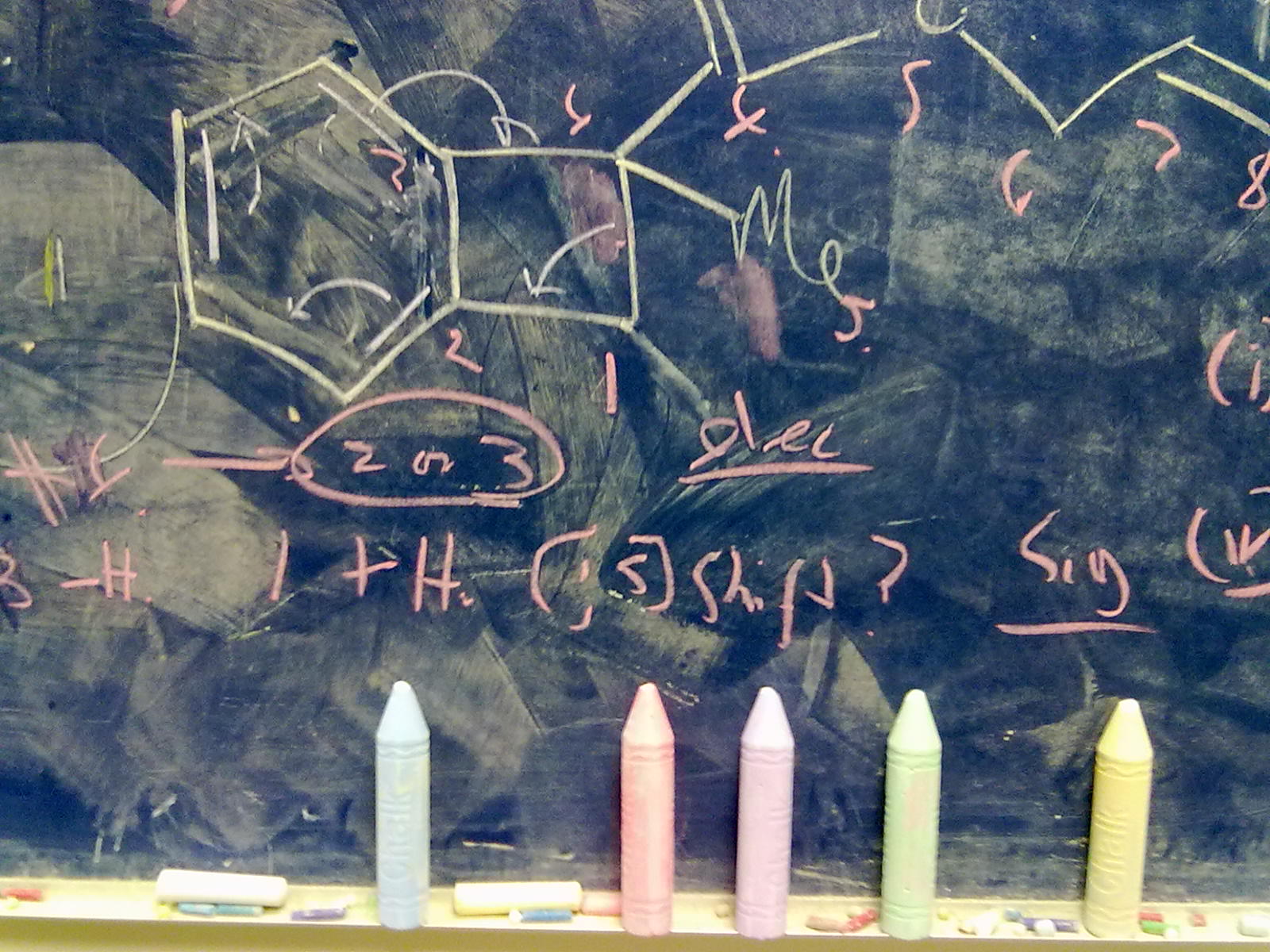
Libraries (and librarians) are evolving rapidly.
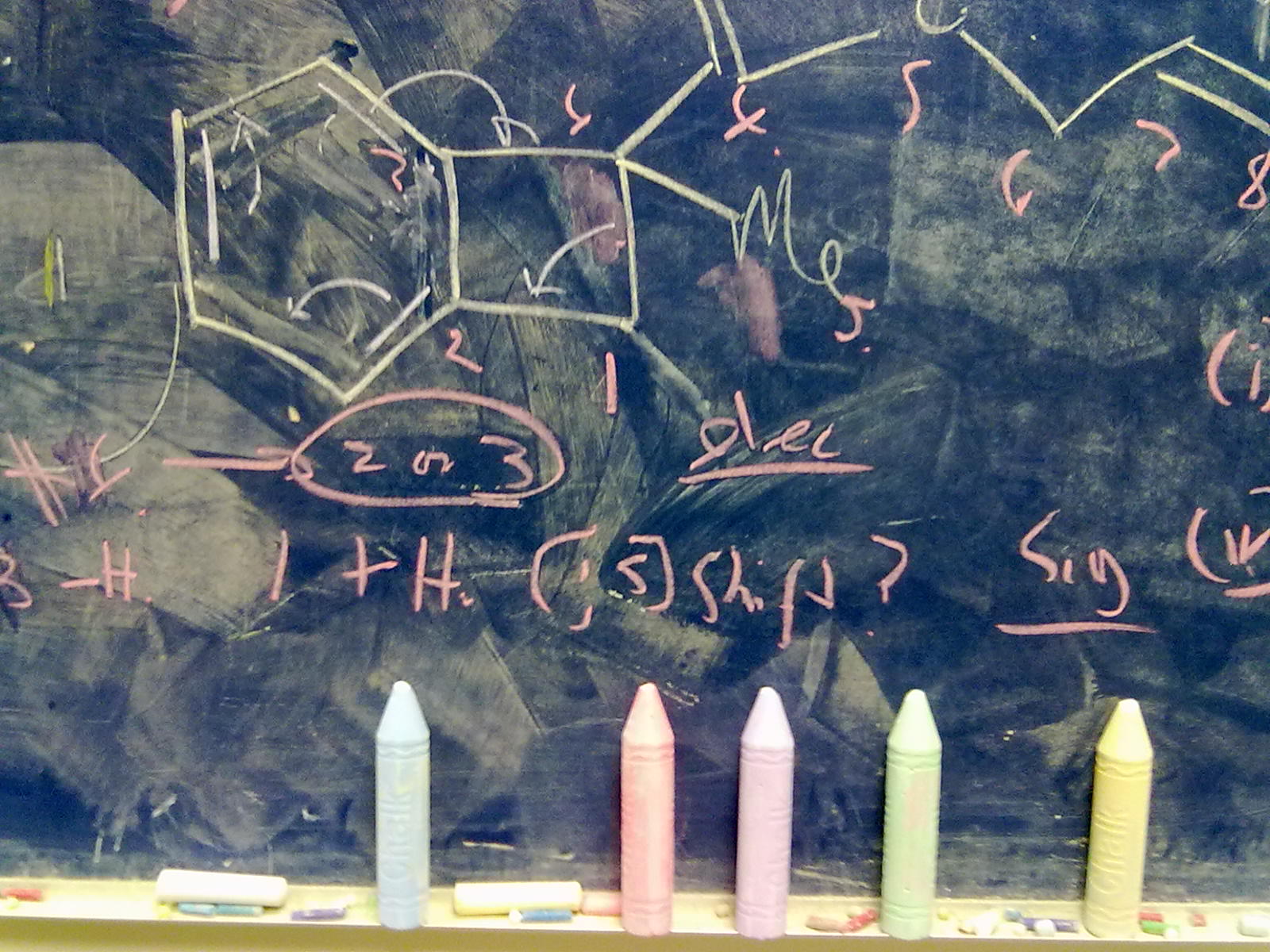
Libraries (and librarians) are evolving rapidly.
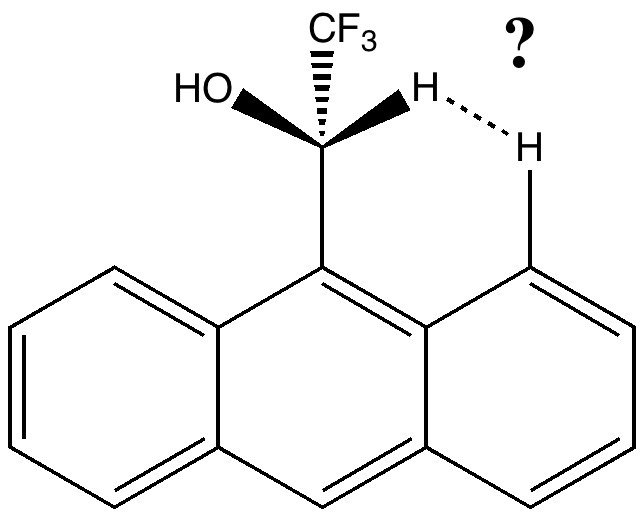
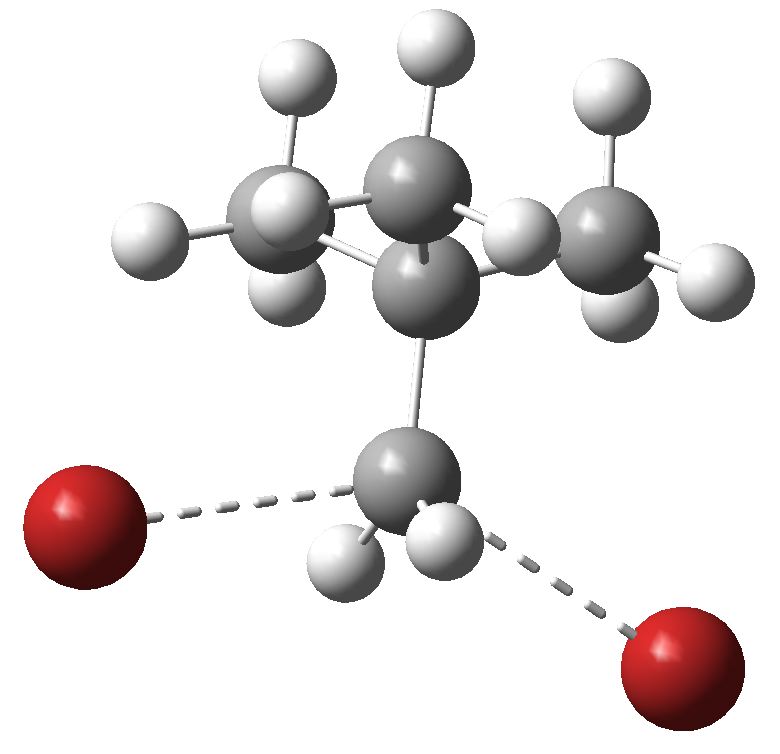
This molecule is not leaving me in peace. It and I first met in 1990 (DO: 10.1039/C39910000765), when we spotted the two unusual π-facial bonds formed when it forms a loose dimer.
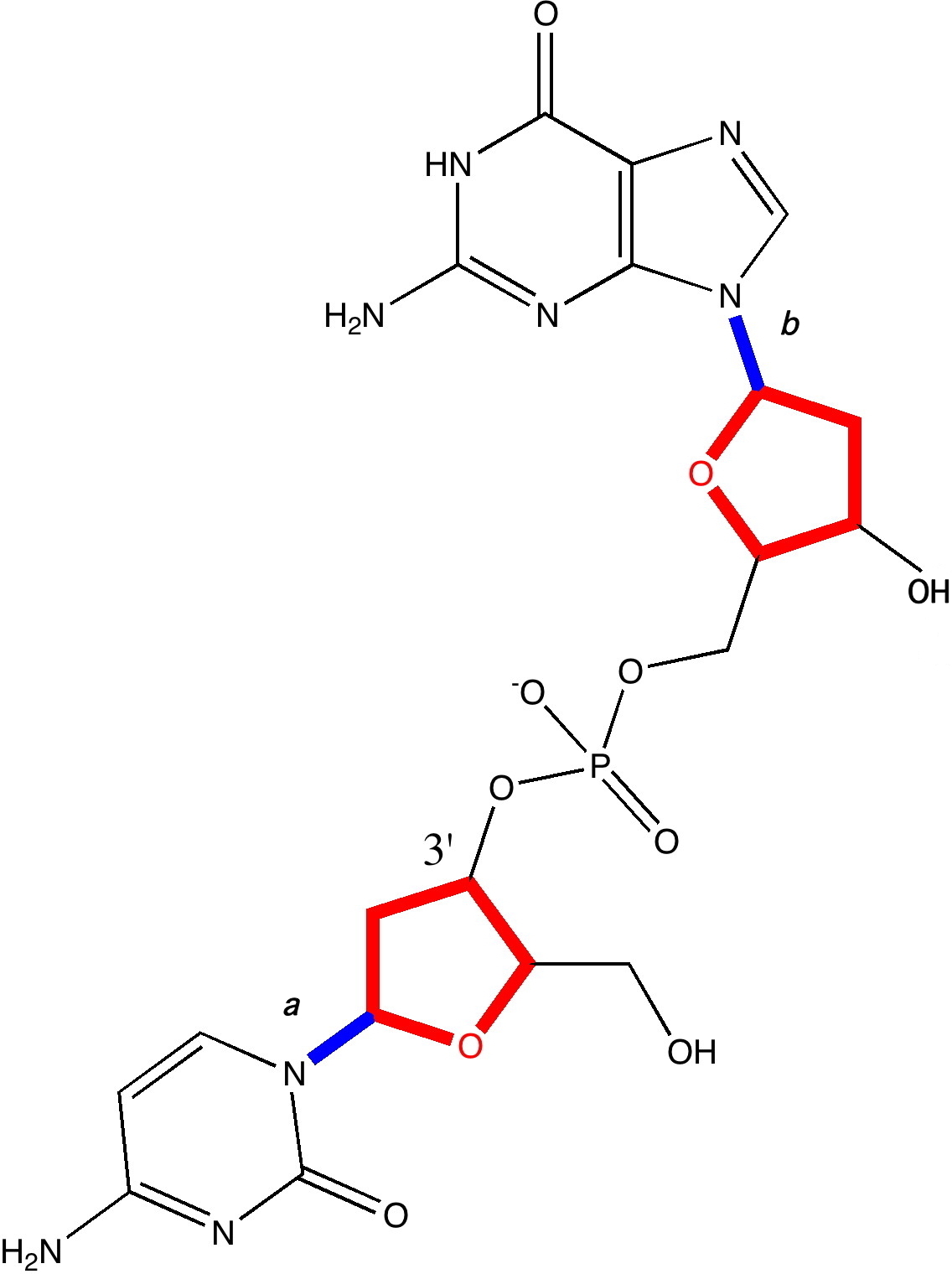
In earlier posts, I alluded to what might make DNA wind into a left or a right-handed helix. Here I switch the magnification of our structural microscope up a notch to take a look at some more inner secrets.
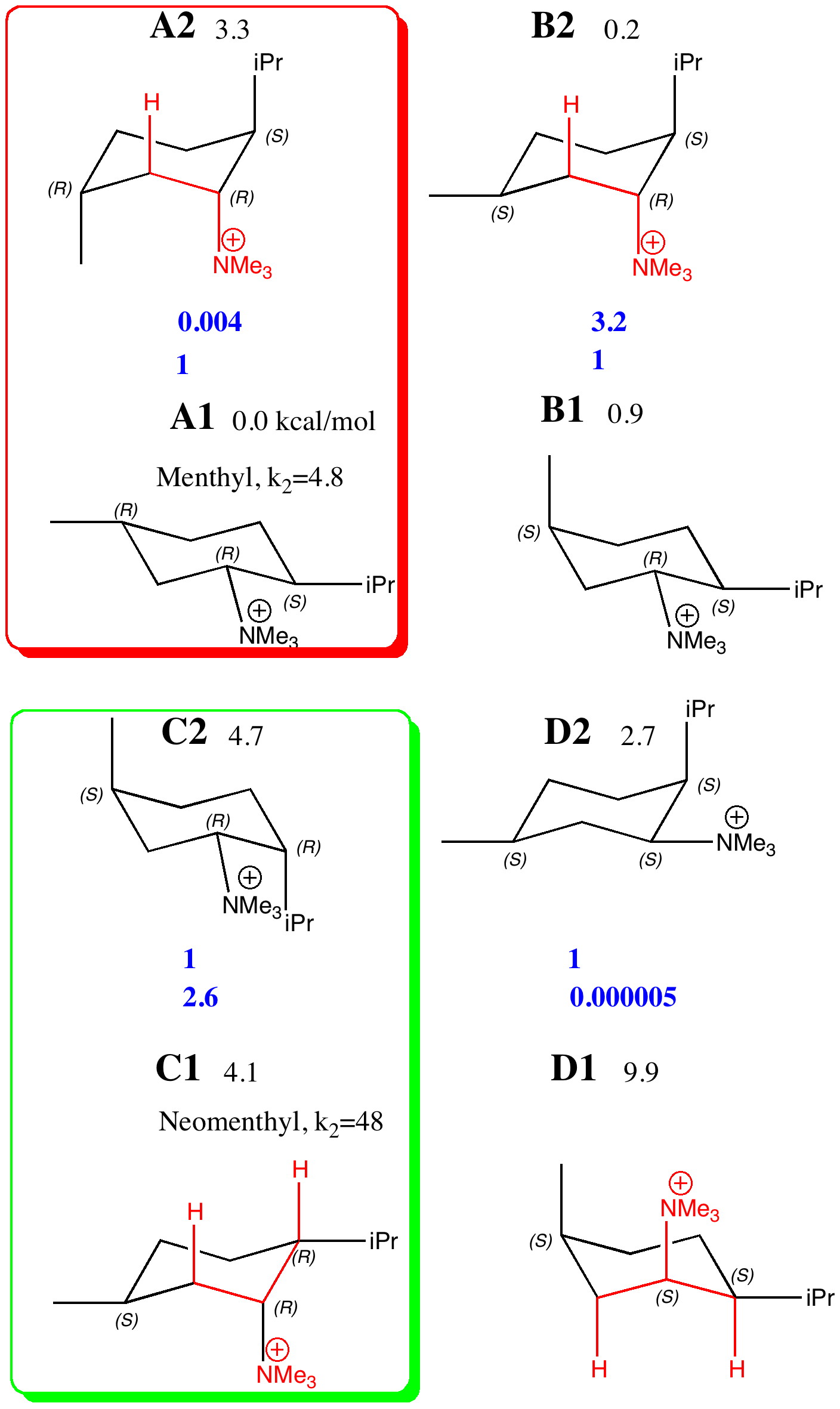
The previous post set out a problem in conformational analysis. Here is my take, which includes an NCI (non-covalent interaction) display as discussed in another post.
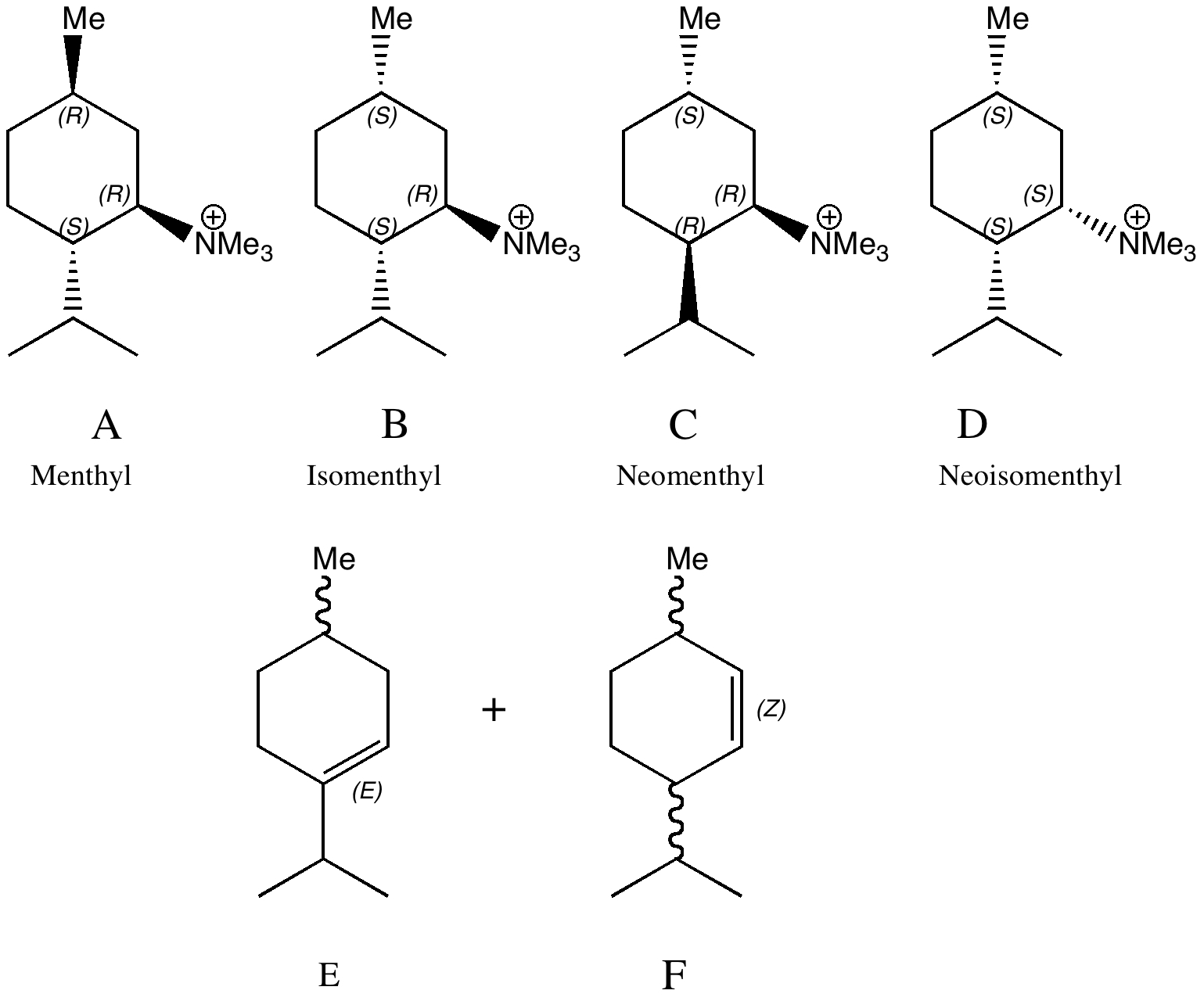
Conformational analysis comes from the classical renaissance of physical organic chemistry in the 1950s and 60s. The following problem is taken from E. D. Hughes and J. Wilby J. Chem.
Introductory organic chemistry invariably features the mechanism of haloalkane solvolysis, and introduces both the Sn1 two-step mechanism, and the Sn2 one step mechanism to students. They are taught to balance electronic effects (the stabilization of carbocations) against steric effects in order to predict which mechanism prevails.
At a recent conference, I talked about what books might look like in the near future, with the focus on mobile devices such as the iPad. I ended by asserting that it is a very exciting time to be an aspiring book author, with one’s hands on (what matters), the content.
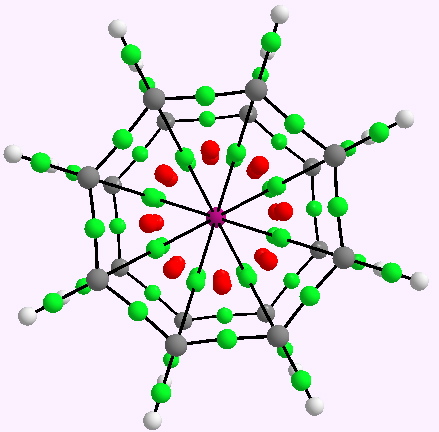
The two previous posts have explored one of the oldest bonding rules (pre-dating quantum mechanics), which postulated that filled valence shells in atoms forming molecules follow the magic numbers 2, 8, 18 and 32. Of the 59,025,533 molecules documented at the instant I write this post, only one example is claimed for the 32-electron class.
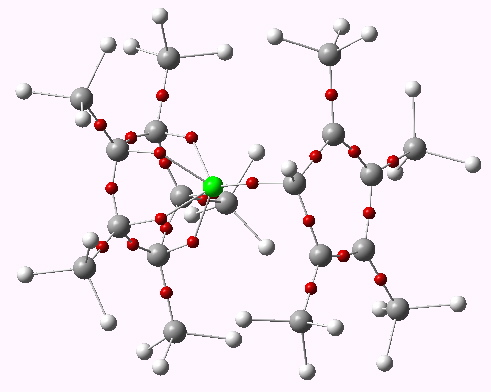
In discussing ferrocene in the previous post, I mentioned Irving Langmuir’s 1921 postulate that filled valence shells in what he called complete molecules would have magic numbers of 2, 8, 18 or 32 electrons (deriving from the sum of terms in 2[1+3+5+7]). The first two dominate organic chemistry of course, whilst the third is illustrated by […]
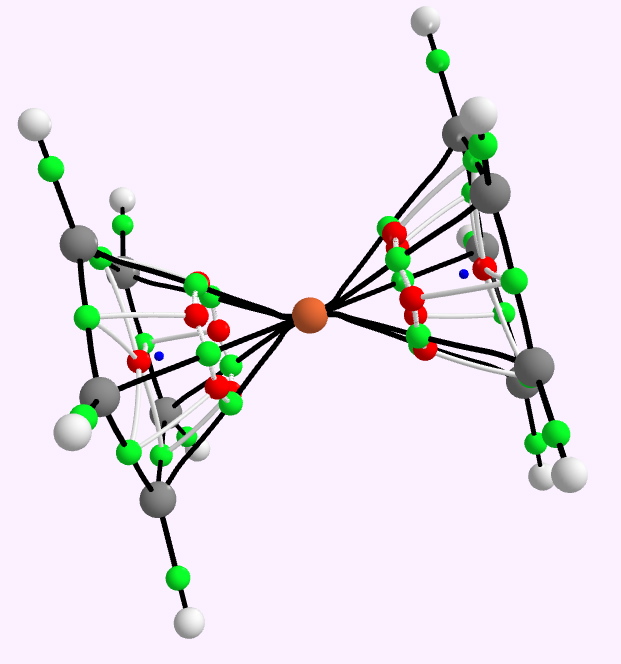
The structure of ferrocene was famously analysed by Woodward and Wilkinson in 1952,, symmetrically straddled in history by Pauling (1951) and Watson and Crick (1953). Quite a trio of Nobel-prize winning molecular structural analyses, all based on a large dose of intuition.
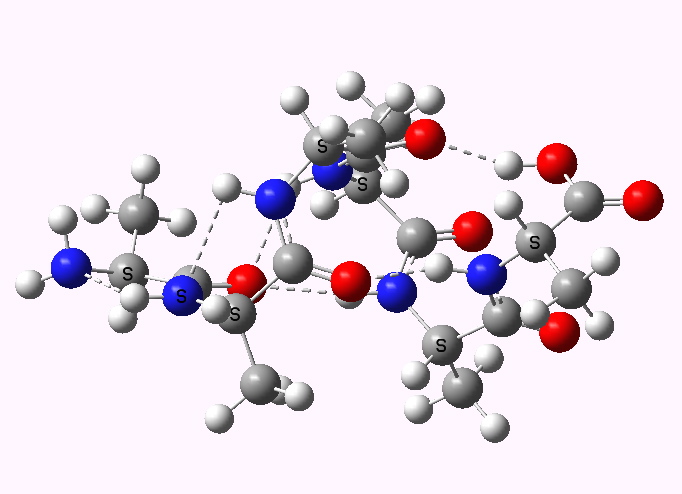
Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry.