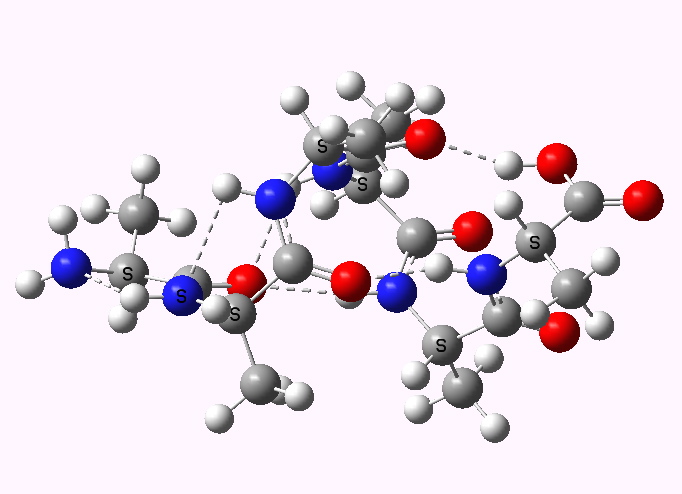
Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry.

Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry.
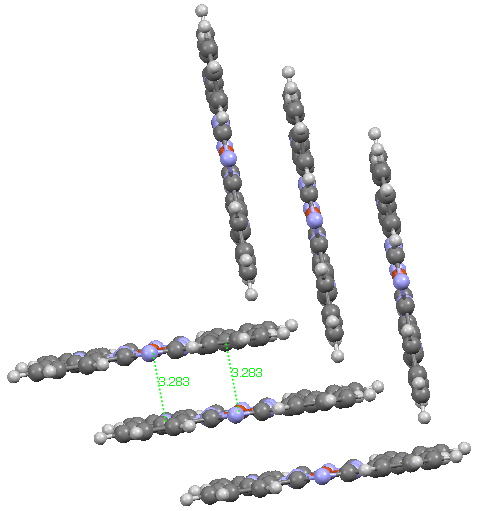
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λmax 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model.
Most scientific theories emerge slowly, over decades, but others emerge fully formed virtually overnight as it were (think Einstein in 1905). A third category is the supernova type, burning brightly for a short while, but then vanishing (almost) without trace shortly thereafter.
I am at the ACS meeting, attending a session on chemistry and the Internet. This post was inspired by Chemicalize, a service offered by ChemAxon, which scans a post like this one, and identifies molecules named. I had previously used generic post taggers, which frankly did not work well in identifying chemical content.
In the previous post I pondered the colour of Monastral blue (copper phthalocyanine). Something did not quite fit, and so I speculated that perhaps some oxidation of the pigment might give a new species.
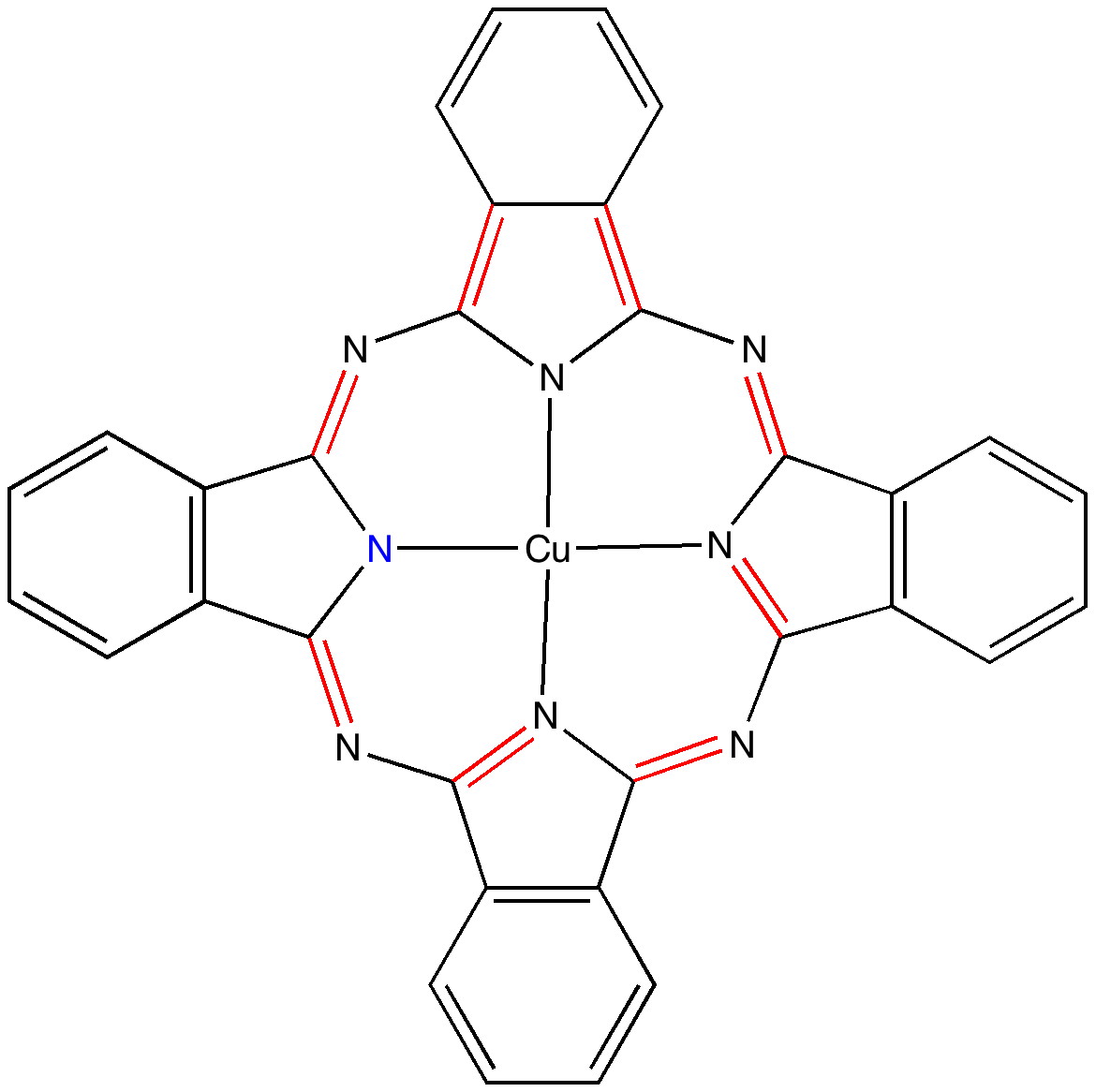
The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else.
In this earlier post, I noted some aspects of the calculated structures of both Z- and B-DNA duplexes. These calculations involved optimising the positions of around 250-254 atoms, for d(CGCG)2 and d(ATAT)2, an undertaking which has taken about two months of computer time!

My colleague Bill Griffith has again come up with another colour challenge: that of the ancient semi-precious stone Lapis Lazuli, mined in the mountains of Afghanistan for more than 6000 years and used by painters in some medieval paintings of the Virgin, the Wilton diptych etc.
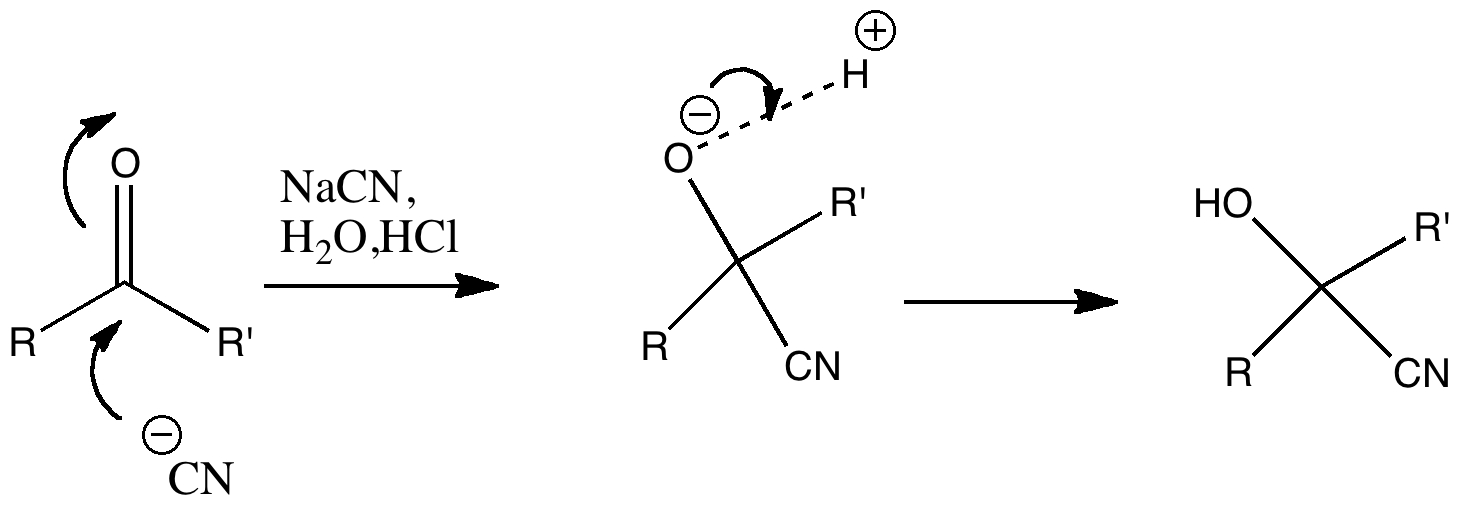
Nucleophilic addition of cyanide to a ketone or aldehyde is a standard reaction for introductory organic chemistry. But is all as it seems? The reaction is often represented as below, and this seems simple enough.
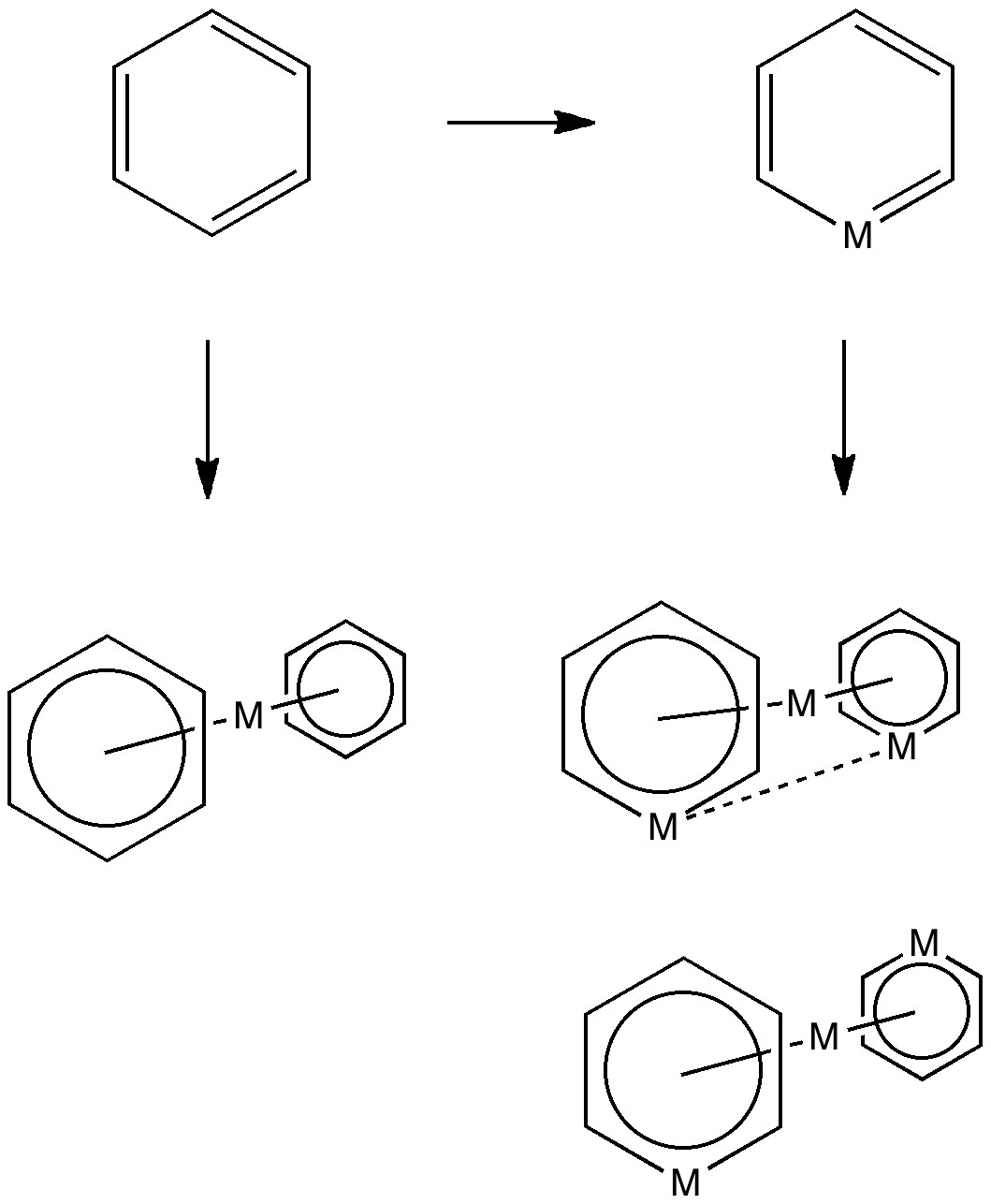
Much like chocolate, some of us metallaholics cannot get enough. So WUQXIP proved an irresistible frolic (DOI: 10.1021/om020789h). Let us start with benzene. It can have metals added in two ways, whilst preserving its essential aromaticity.

One of my chemical heroes is William Perkin, who in 1856 famously (and accidentally) made the dye mauveine as an 18 year old whilst a student of August von Hofmann, the founder of the Royal College of Chemistry (at what is now Imperial College London). Perkin went on to found the British synthetic dyestuffs and perfumeries industries.