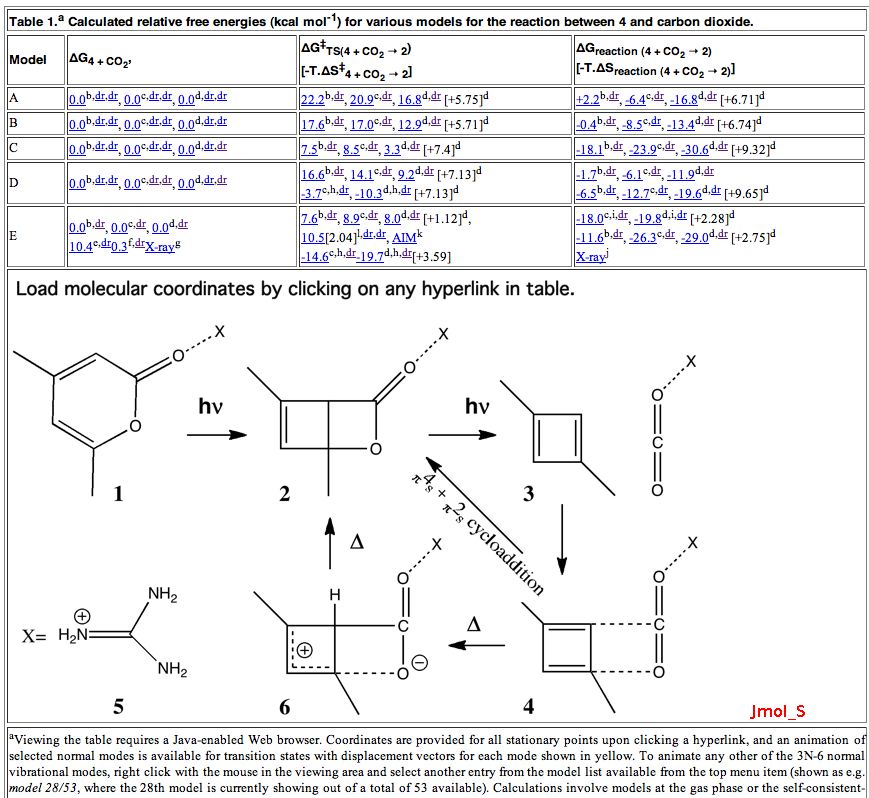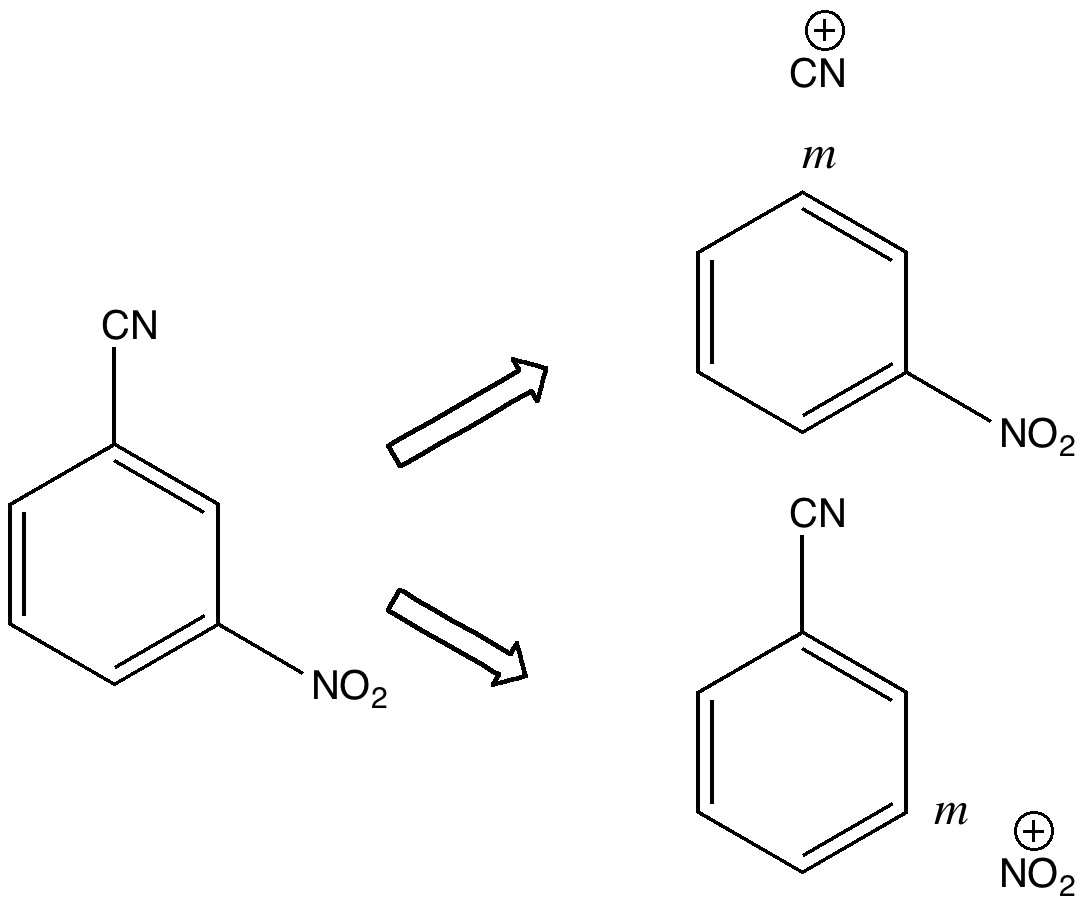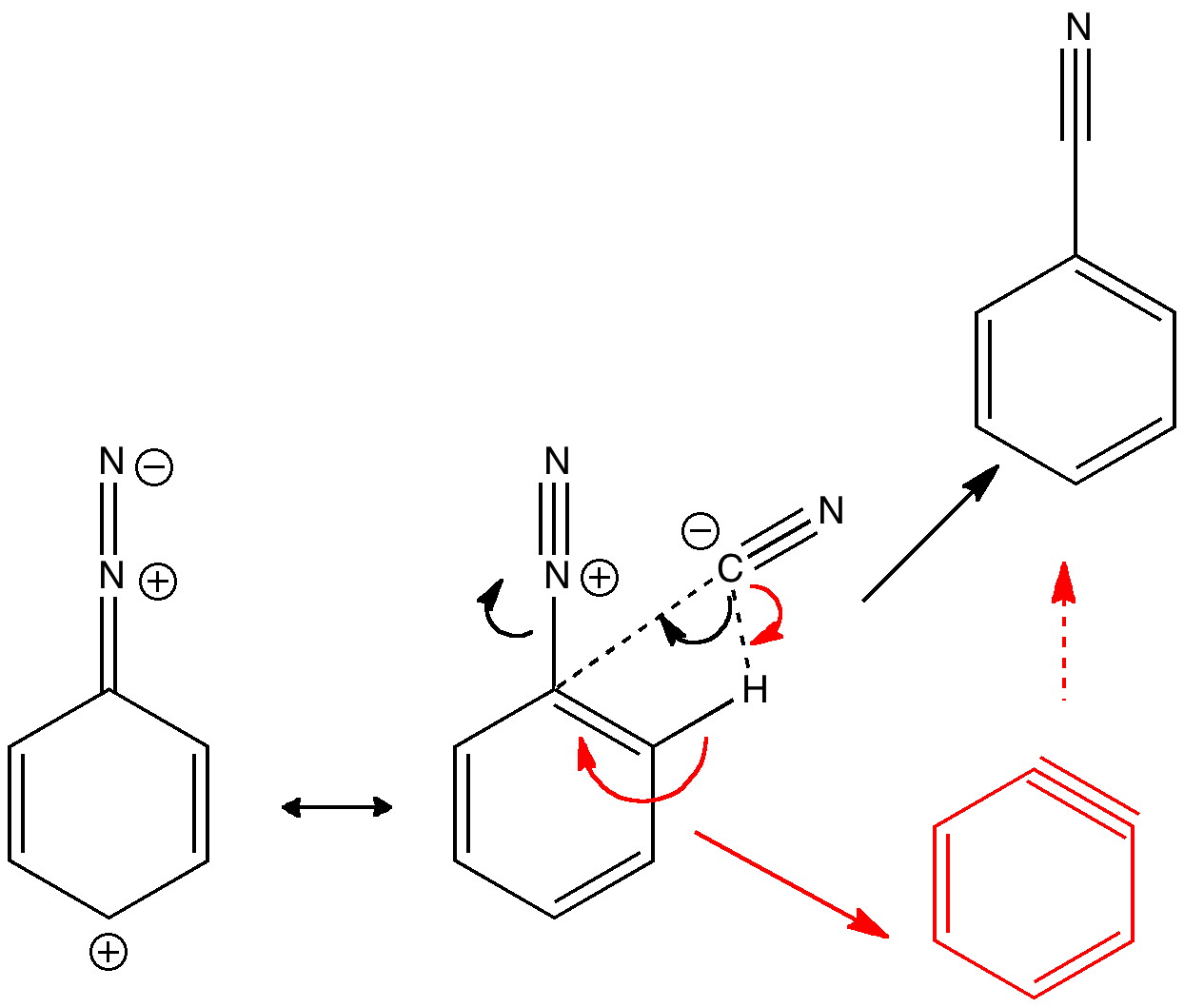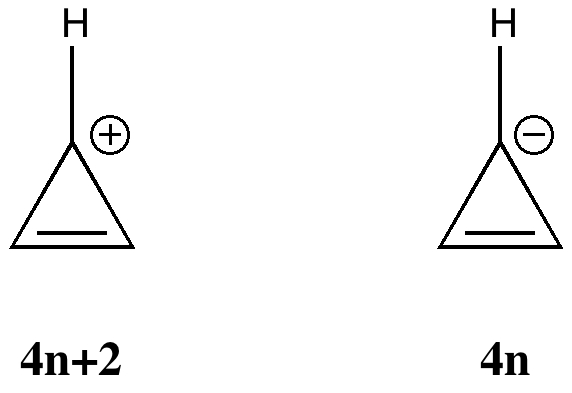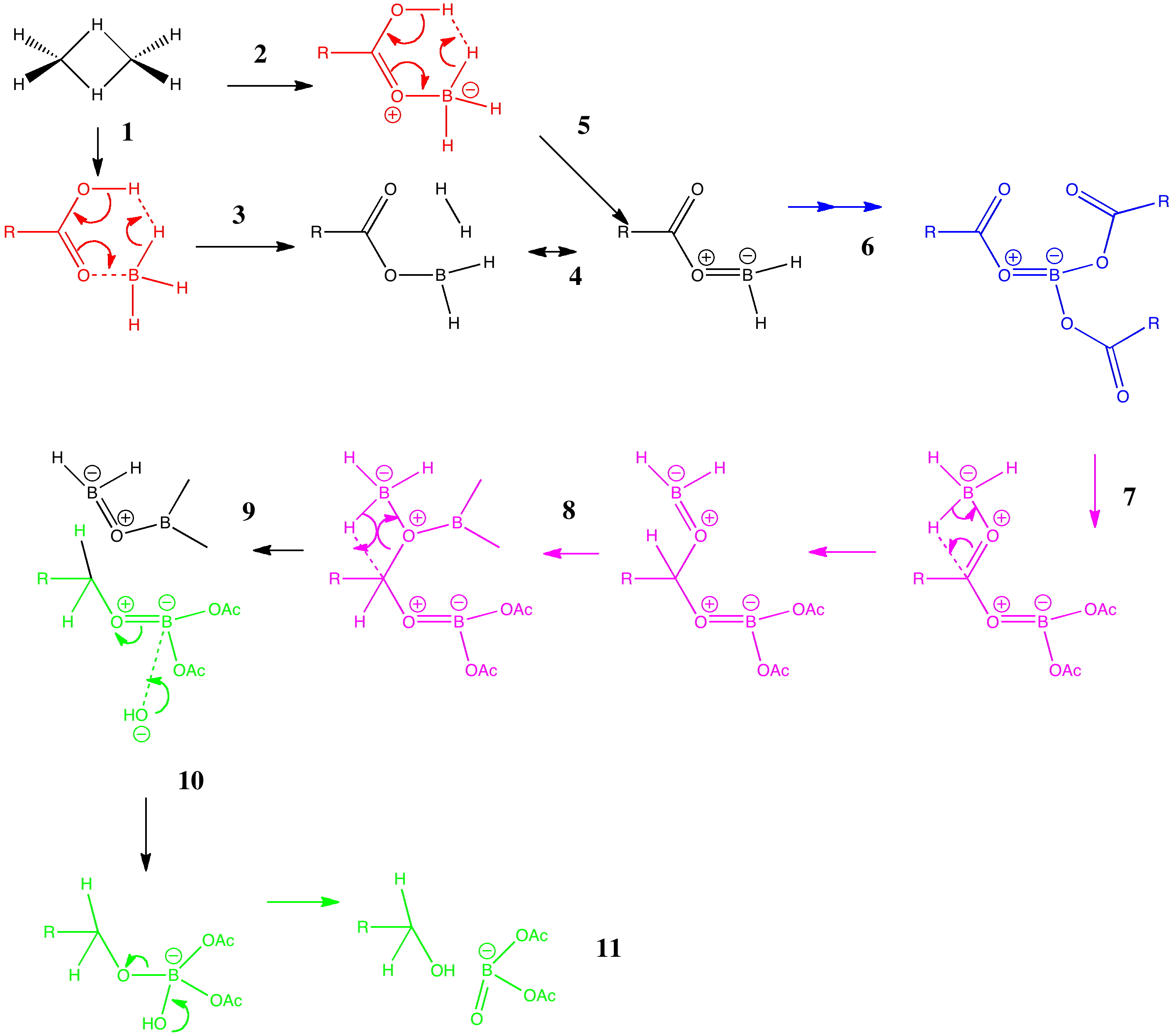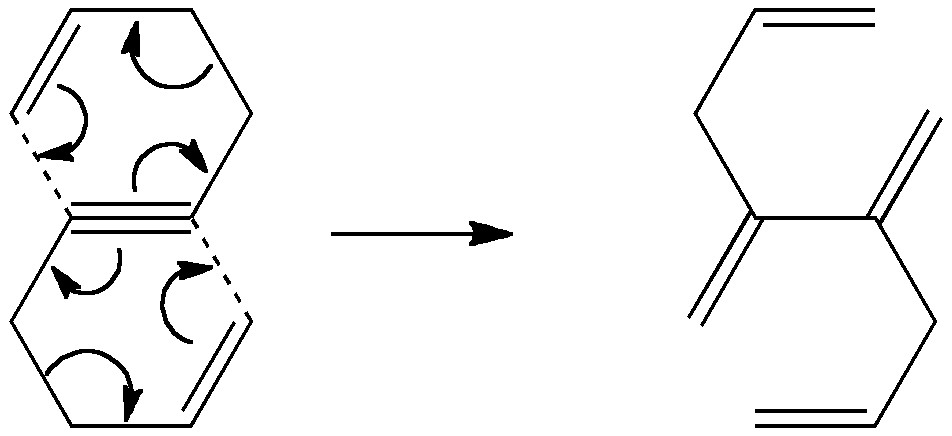
Is there a preferred pack size for electrons on the move? Or put less flamboyantly, is there an optimum, and a maximum number of arrows (electron pairs) that one might push in revealing the mechanism of a concerted reaction? A sort of village-instinct for electrons.