This editorial from Nature is a timely reminder of the importance of data.
Some time ago in 2010, I showed a chemical problem I used to set during university entrance interviews. It was all about pattern recognition and how one can develop a hypothesis based on this.
One of the important aspects of chemical reaction mechanisms is the order in which things happen. More specifically, the order in which bonds make or break when there are more than two involved in undertaking a reaction.
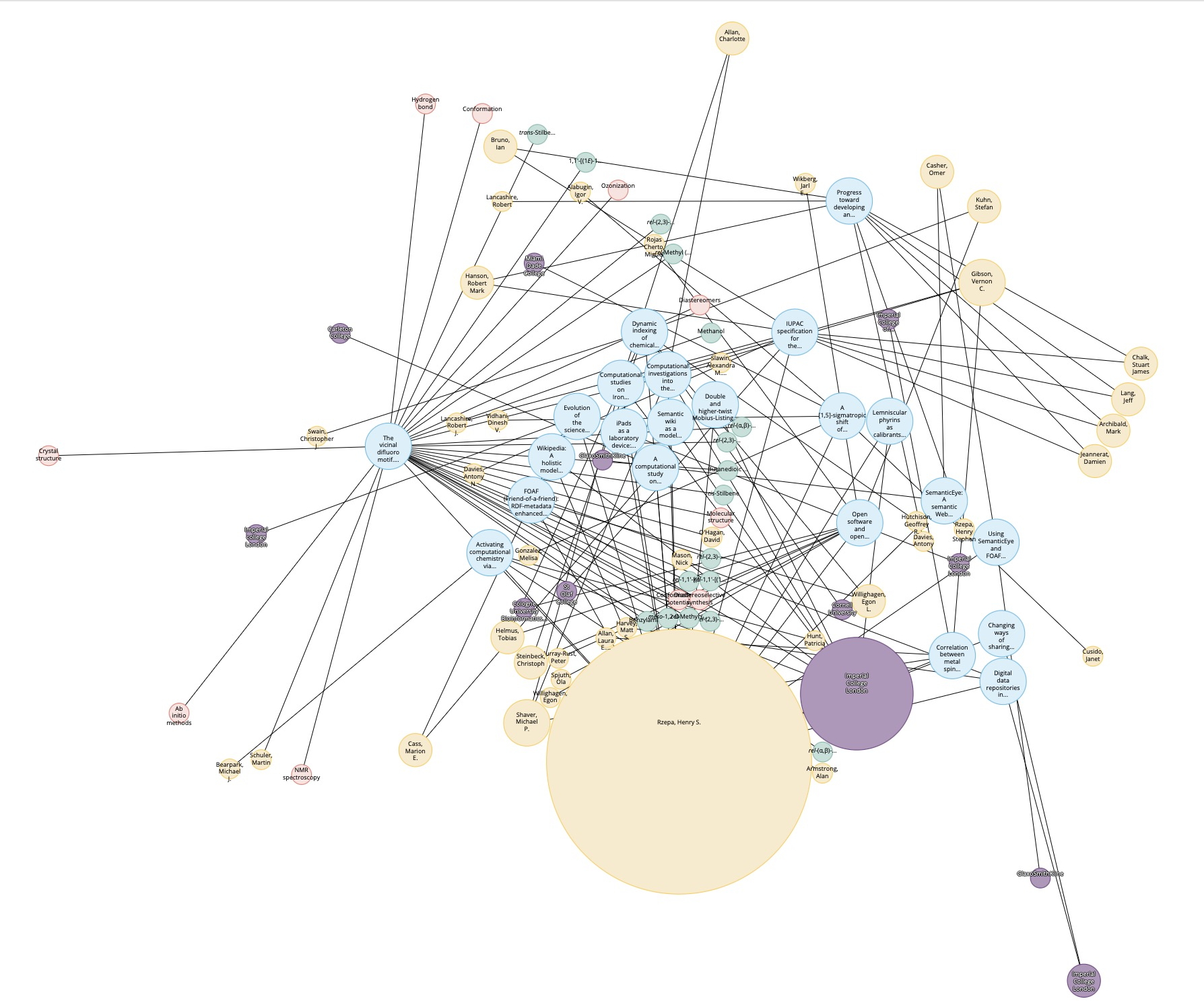
Science frequently works by people making connections between related (or even apparently unrelated) concepts or data. There are many ways of helping people make these connections – attending a conference or seminar, searching journals for published articles and nowadays also searching for data are just a few examples.
Nature has produced most natural molecules as chiral objects, which means the molecule can come in two enantiomeric forms, each being the mirror image of the other.
I have previously looked at the pigments used to colour the Book of Kells, which dates from around 800 AD and which contained arsenic sulfide as the yellow colourant. The Bayeaux tapestry is a later embroidery dating probably from around 1077 and here the colours are based entirely on mordanted natural dyes.
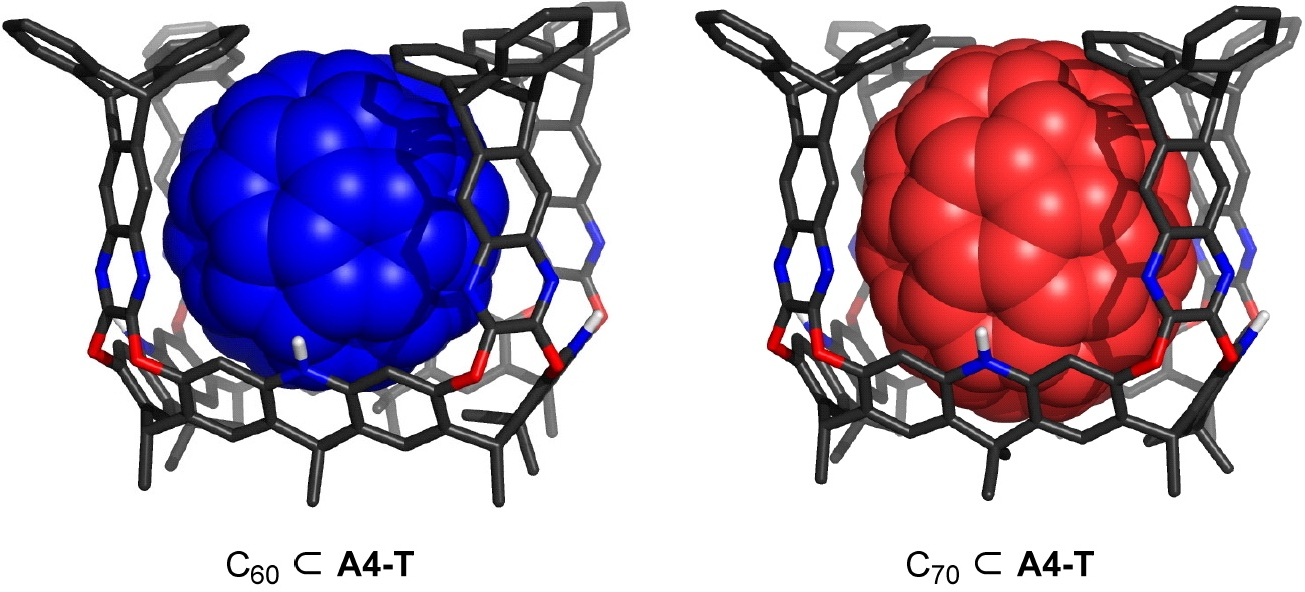
In the previous post, I discussed how data associated with two of the candidates for molecules of the year – 2022 could be retrieved and then used to inspect their three dimensional structures. Here I focus on the ultra large cavitands recently reported.

The list of molecules of the year is out now at C&E News (but you have to have an account to view the list, unlike previous years).♣ These three caught my eye:
The topic of dicarbon, C2, has been discussed here for a few years now. It undoubtedly would be a gas!
What’s a Journal For? This debate has been raging ever since preprint servers were introduced as far back as 1991!
Sometimes you come across a reaction which is so simple in concept that you wonder why it took so long to be accomplished in practice.