
The Cheshire cat in Alice’s Adventures in Wonderland comes and goes at will, and engages Alice with baffling philosophical points. Chemical bonds are a bit like that too.

The Cheshire cat in Alice’s Adventures in Wonderland comes and goes at will, and engages Alice with baffling philosophical points. Chemical bonds are a bit like that too.
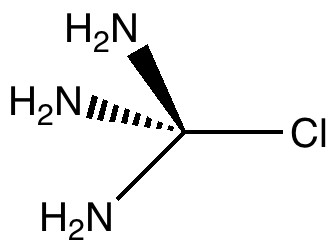
Car transmissions come in two types, ones with fixed ratio gears, and ones which are continuously variable. When it comes to chemical bonds, we tend to think of them as being very much of the first type. Bonds come in fixed ratios; single, aromatic, double, triple, etc.

Stereo-induction is, lets face it, a subtle phenomenon.
A conjugated, (apparently) aromatic molecular trefoil might be expected to have some unusual, if not extreme properties. Here some of these are explored.

Something important happened in chemistry for the first time about 100 years ago. A molecule was built (nowadays we would say synthesized) specifically for the purpose of investigating a theory.

In the first part of the post on this topic, I described how an asymmetric sulfoxide could be prepared as a pure enantiomer using a chiral oxygen transfer reagent. In the second part, we now need to deliver a different group, cyano, to a specific face of the previously prepared sulfoxide-imine.

The assembly of a molecule for a purpose has developed into an art form, one arguably (chemists always argue) that is approaching its 100th birthday (DOI: 10.1002/cber.191104403216) celebrating Willstätter’s report of the synthesis of cyclo-octatetraene. Most would agree it reached its most famous achievement with Woodward’s synthesis of quinine (DOI: 10.1021/ja01221a051) in 1944.
In this previous blog post I wrote about one way in which we have enhanced the journal article.

Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found […]
Since I have gotten into the habit of quoting some of my posts in other contexts, I have started to also archive them using WebCite.
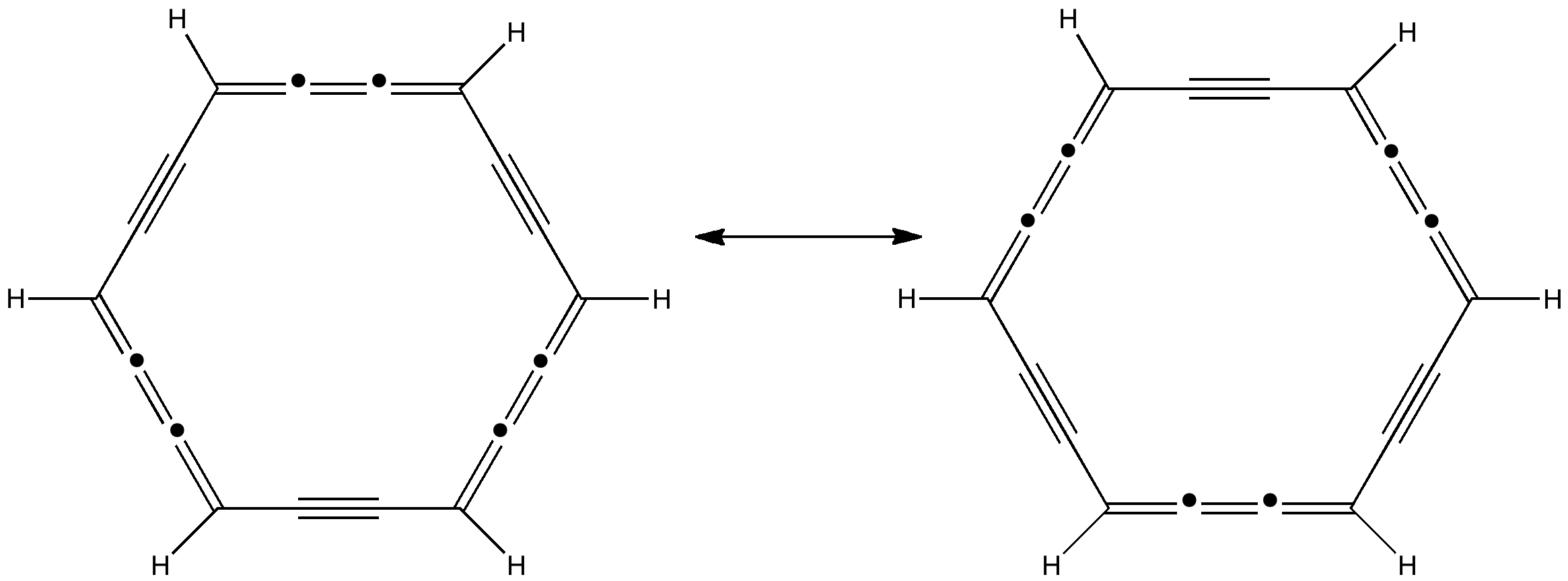
Some molecules, when you first see them, just intrigue. So it was with carbobenzene, the synthesis of a derivative of which was recently achieved by Remi Chauvin and co-workers (DOI: 10.1002/chem.200601193). Two additional carbon atoms have been inserted into each of the six C-C bonds in benzene.