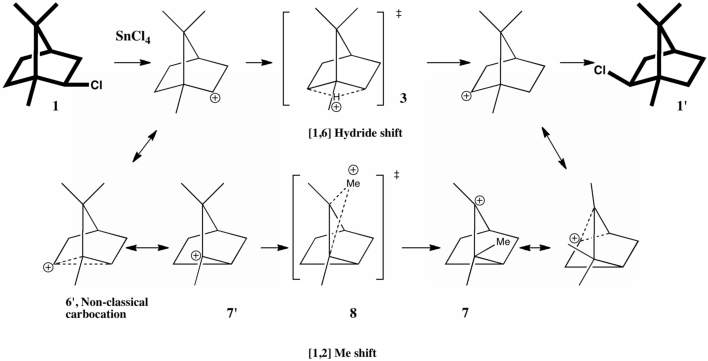
Like benzene, its fully saturated version cyclohexane represents an icon of organic chemistry. By 1890, the structure of planar benzene was pretty much understood, but organic chemistry was still struggling somewhat to fully embrace three rather than two dimensions.