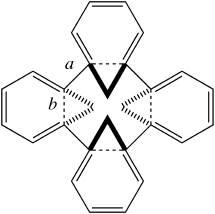
In the previous post, it was noted that Möbius annulenes are intrinsically chiral, and should therefore in principle be capable of resolution into enantiomers. The synthesis of such an annulene by Herges and co-workers was a racemic one; no attempt was reported at any resolution into such enantiomers.