Sometimes you come across a reaction which is so simple in concept that you wonder why it took so long to be accomplished in practice.
The term bispericyclic reaction was famously coined by Caramella et al in 2002 to describe the unusual features of the apparently innocuous dimerisation of cyclopentadiene.
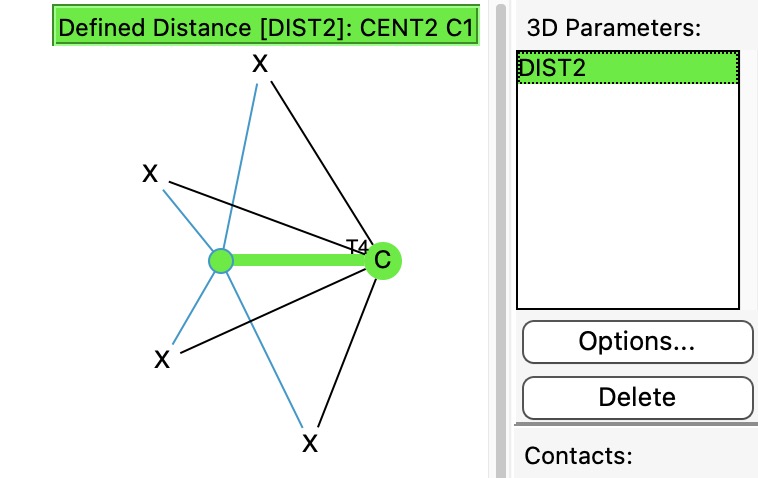
In previously asking what the largest angle subtended at four-coordinate carbon might be, I noted that as the angle increases beyond 180°, the carbon becomes inverted, or hemispherical (all four ligands in one hemisphere). So what does a search for this situation reveal in the CSD?
Four-coordinate carbon normally adopts a tetrahedral shape, where the four angles at the carbon are all 109.47°. But how large can that angle get, and can it even get to be 180°?
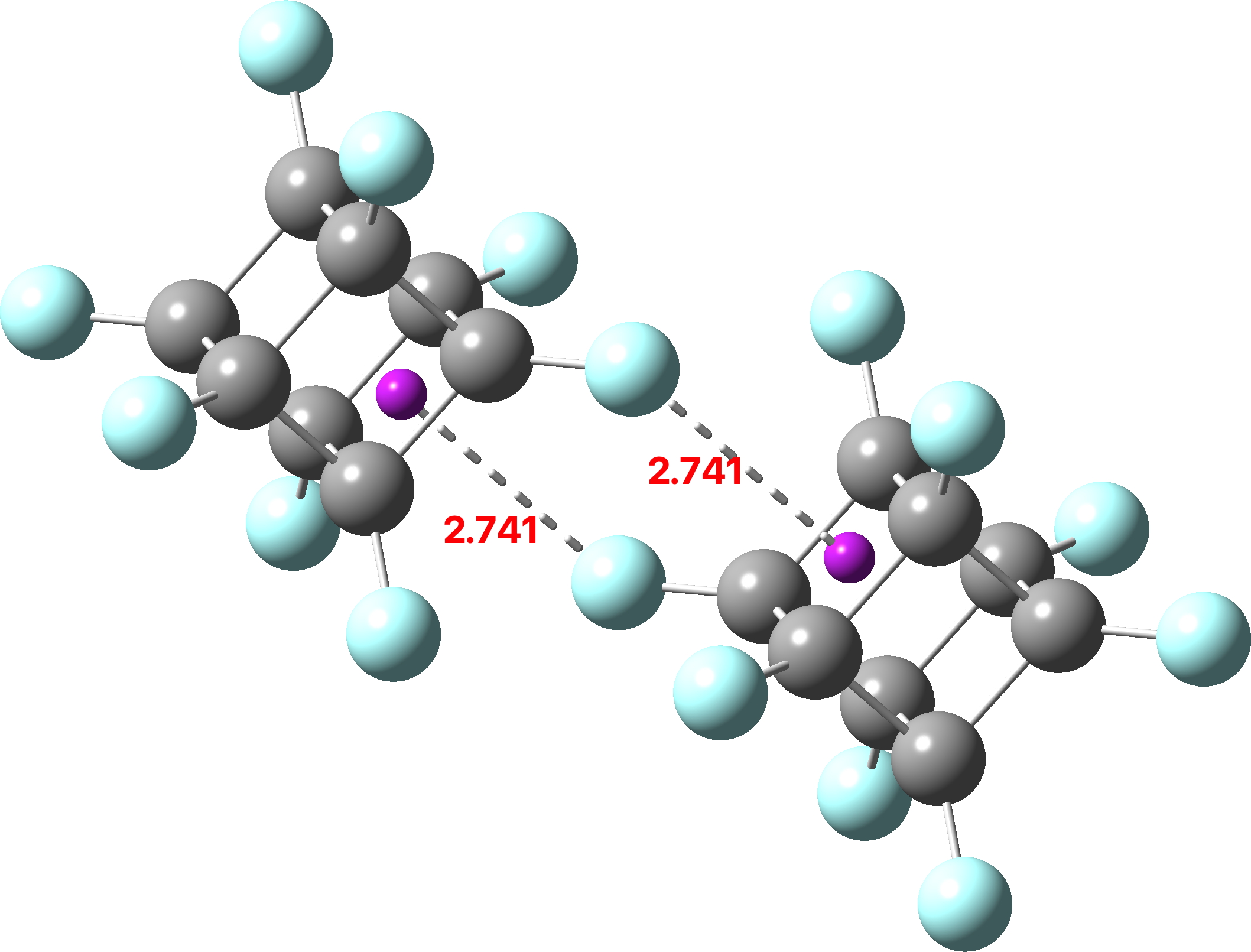
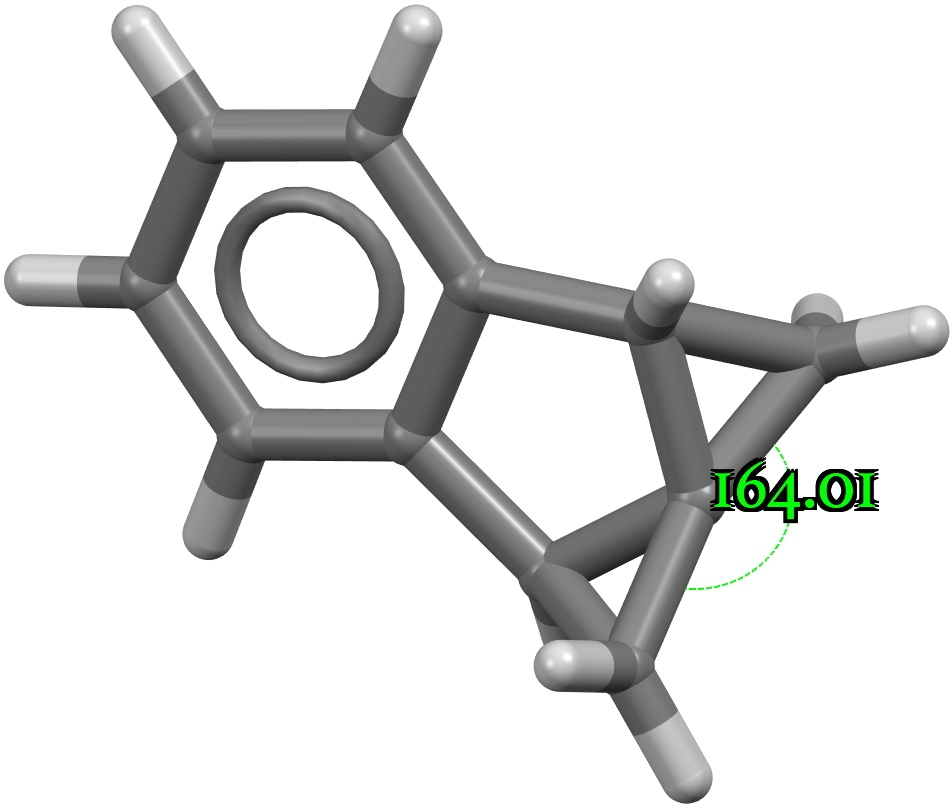
The recently reported synthesis of octafluorocubane established a sublimation point as 168.1–177.1°C (a melting point was not observed). In contrast, the heavier perfluoro-octane has an m.p. of -25°C. Why the difference? Firstly, the crystal structure is shown below, albeit as a dimer rather than a periodic lattice (click on image to obtain 3D coordinates).
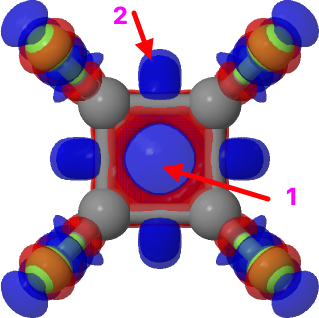
Derek Lowe reports the story that the recently synthesized octafluorocubane can absorb one electron to form a radical anion – an electron in a cube. So I thought it would be fun to compute exactly where that electron sits!
A previous post was triggered by Peter alerting me that interactive electronic supporting information (IESI) we had submitted to a journal in 2005 appeared to be strangely missing from the article landing page.
Previously, I looked at autocatalytic mechanisms where the carboxyl group of an oxetane-carboxylic acid could catalyse its transformation to a lactone, finding that a chain of two such groups were required to achieve the result.
Having established a viable model for the unexpected isomerism of oxetane carboxylic acids to lactones, and taken a look at a variation in the proton transfer catalyst needed to accomplish the transformation, I now investigate the substrate itself.
Previously, a mechanism with a reasonable predicted energy was modelled for the isomerisation of an oxetane carboxylic acid to a lactone by using two further molecules of acid to transfer the proton and in the process encouraging an Sn2 reaction with inversion to open the oxetane ring.
In 2006 we published an article illustrating various types of pseudorotations in small molecules. It’s been cited 20 times since then, so reasonable interest! We described rotations known as Lever and Turnstile as well as the better known Berry mode.