These posts contain the computed potential energy surfaces for a fair few “text-book” reactions. Here I chart the course of the cyclopropanation of alkenes using the Simmons-Smith reagent,[cite]10.1021/ja01552a080[/cite] as prepared from di-iodomethane using zinc metal insertion into a C-I bond. Two reactions it can be compared with are the epoxidation of ethene using a peracid and dichlorocyclopropanation.
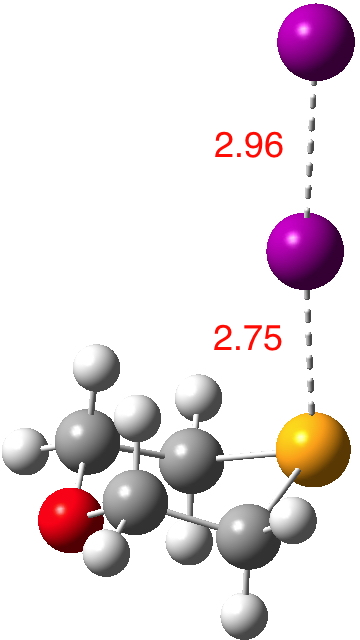
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[cite]10.1021/ic50038a006[/cite]. The features of interest include: The six-membered ring is in the chair conformation.

Nitrogen tri-iodide, or more accurately the complex between it and ammonia ranks amongst the oldest known molecules (1812). I became familiar with it around the age of 12-13, in an era long gone when boys (and very possibly girls too) were allowed to make such substances in their parent’s back gardens ‡ and in fact in the school science laboratory, † an experiment which earned me a personal request to visit the head teacher.

Pursuing the topic of halogen bonds, the system DABCO (a tertiary dibase) and iodine form an intriguing complex. Here I explore some unusual features of the structure HEKZOO[cite]10.5517/CCYJN03[/cite] as published in 2012[cite]10.1021/cg300669t[/cite] and ask whether the bonding between the donor (N) and the acceptor (I-I) really is best described as a “non-covalent-interaction” (NCI) or not.
Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom ( X , acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A . They are superficially surprising, since both D and X look like electron rich species.
In London, one has the pleasures of attending occasional one day meetings at the Burlington House, home of the Royal Society of Chemistry. On November 5th this year, there was an excellent meeting on the topic of *Challenges in Catalysis, *and you can see the speakers and (some of) their slides here.
Solvolytic mechanisms are amongst the oldest studied, but reproducing their characteristics using computational methods has been a challenging business.
Egon Willighagen recently gave a presentation at the RSC entitled “The Web – what is the issue” where he laments how little uptake of web technologies as a “*channel for communication of scientific knowledge and data” *there is in chemistry after twenty years or more. It caused me to ponder what we were doing with the web twenty years ago.

We are approaching 1 million recorded crystal structures (actually, around 716,000 in the CCDC and just over 300,00 in COD). One delight with having this wealth of information is the simple little explorations that can take just a minute or so to do. This one was sparked by my helping a colleague update a set of interactive lecture demos dealing with stereochemistry.
This second report highlights two “themes”, or common ideas that seem to emerge spontaneously from diversely different talks. Most conferences do have them. The first is “ embedding ”, which in this context means treating different parts of a probably complex molecular system at different levels of theory.

I am attending a conference. Plenaries at such events can sometimes provide interesting pointers on things to come (and sometimes they simply point to things past). At WATOC2014 in Santiago Chile, the first plenary was by Paul Ayers with the impressive title “ Concepts for organising chemical knowledge ” which certainly sounds as if it is pointing forward!