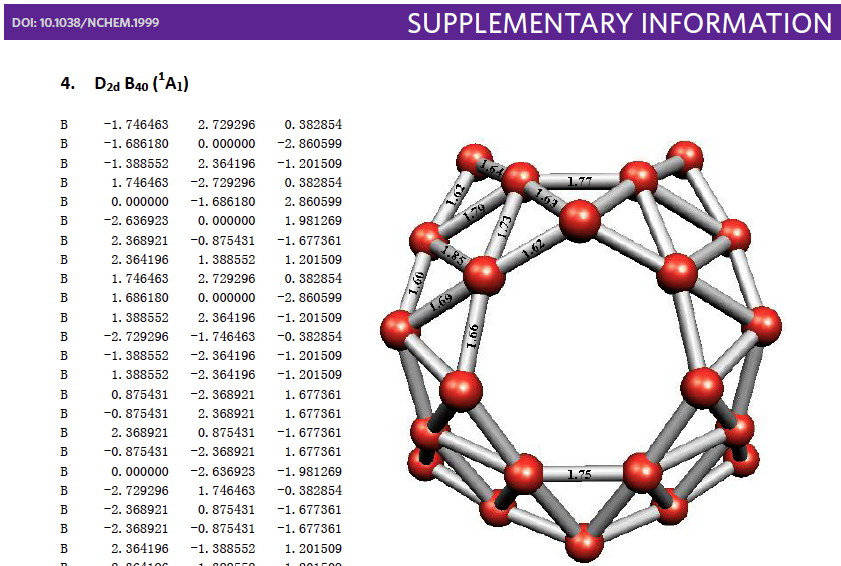
I am attending a conference. Plenaries at such events can sometimes provide interesting pointers on things to come (and sometimes they simply point to things past). At WATOC2014 in Santiago Chile, the first plenary was by Paul Ayers with the impressive title “ Concepts for organising chemical knowledge ” which certainly sounds as if it is pointing forward!