So much to do, so little time to do it. That is my excuse at least.
A game one can play with pericyclic reactions is to ask students to identify what type a given example is. So take for example the reaction below.
As my previous post hints, I am performing my annual spring-clean of lecture notes on pericyclic reactions. Such reactions, and their stereochemistry, are described by a set of selection rules. I am always on the lookout for a simple example which can most concisely summarise these rules.

When I first started giving lectures to students, it was the students themselves that acted as human photocopiers, faithfully trying to duplicate what I was embossing on the lecture theatre blackboard with chalk. How times have changed!
Text books will announce that during aromatic electrophilic substitution, aromaticity is lost by the formation of a Wheland intermediate (and regained by eliminating a proton). Is that entirely true?
I have several times used arrow pushing on these blogs. But since the rules for this convention appear to be largely informal, and there appears to be no definitive statement of them, I thought I would try to produce this for our students. This effort is here shared on my blog.
This is one of those topics that seems to crop up every three years or so. Since then, new versions of operating systems, new versions of programs, mobile devices and perhaps some progress?
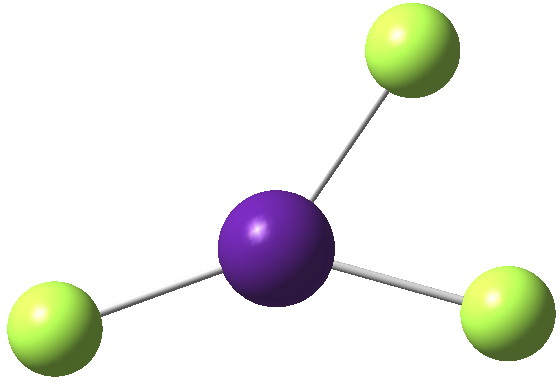
Mercury (IV) tetrafluoride attracted much interest when it was reported in 2007 as the first instance of the metal being induced to act as a proper transition element (utilising d-electrons for bonding) rather than a post-transition main group metal (utilising just s-electrons) for which the HgF2 dihalide would be more normal (“Is mercury now a […]
Not long ago, I described a cyclic carbene in which elevating the carbene lone pair into a π-system transformed it from a formally 4n-antiaromatic π-cycle into a 4n+2 aromatic π-cycle. From an entirely different area of chemistry, another example of this behaviour emerges; Schreiner’s trapping and reactions of t-butyl-hydroxycarbene, as described on Steve Bachrach’s blog.
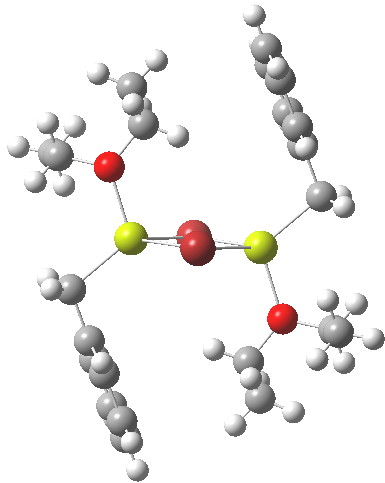
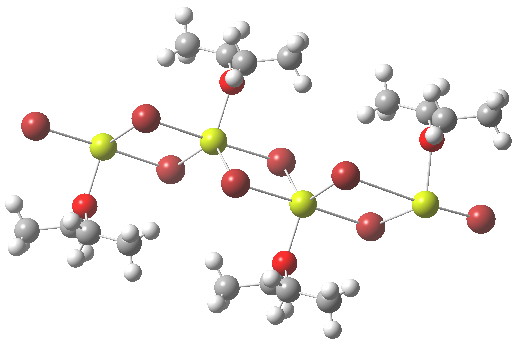
In the previous post I mentioned in passing the Grignard reagent benzyl magnesium bromide as having tetrahedral coordination at Mg. But I have now noticed, largely through spotting Steve Bachrach’s post on “Acene dimers – open or closed?” another geometric effect perhaps worthy of note, certainly one not always noted in the past;
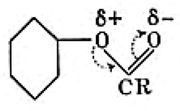
The following is a short question in a problem sheet associated with introductory organic chemistry.