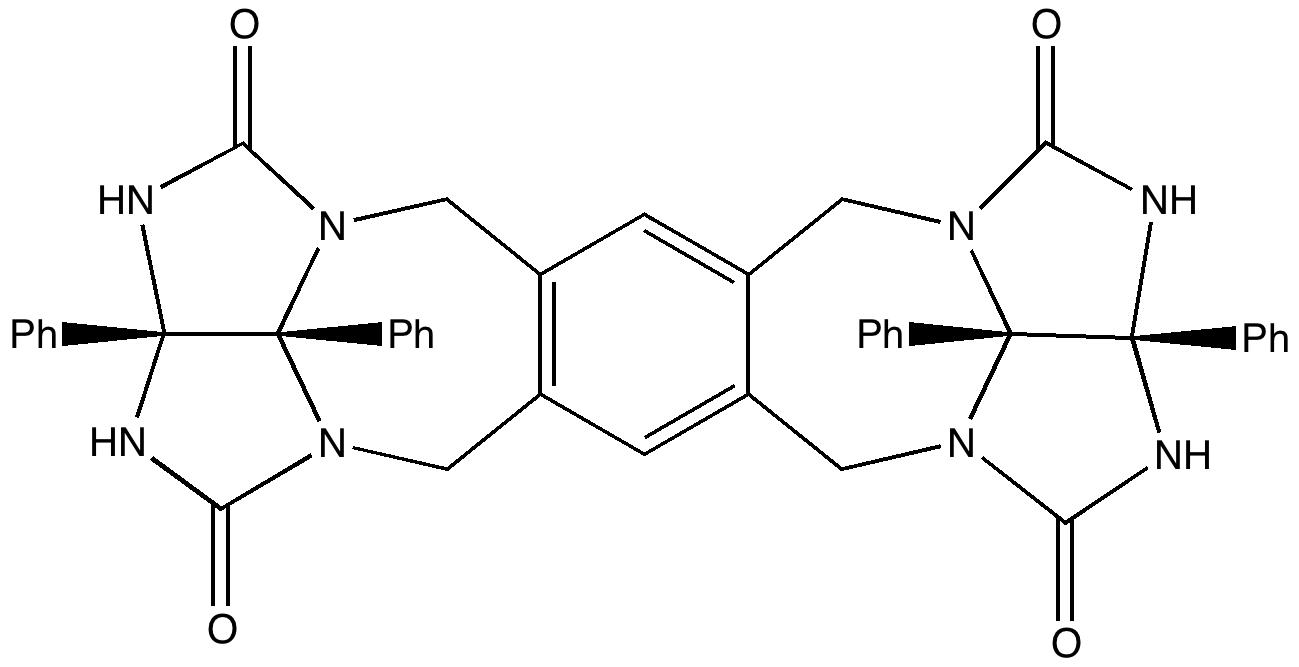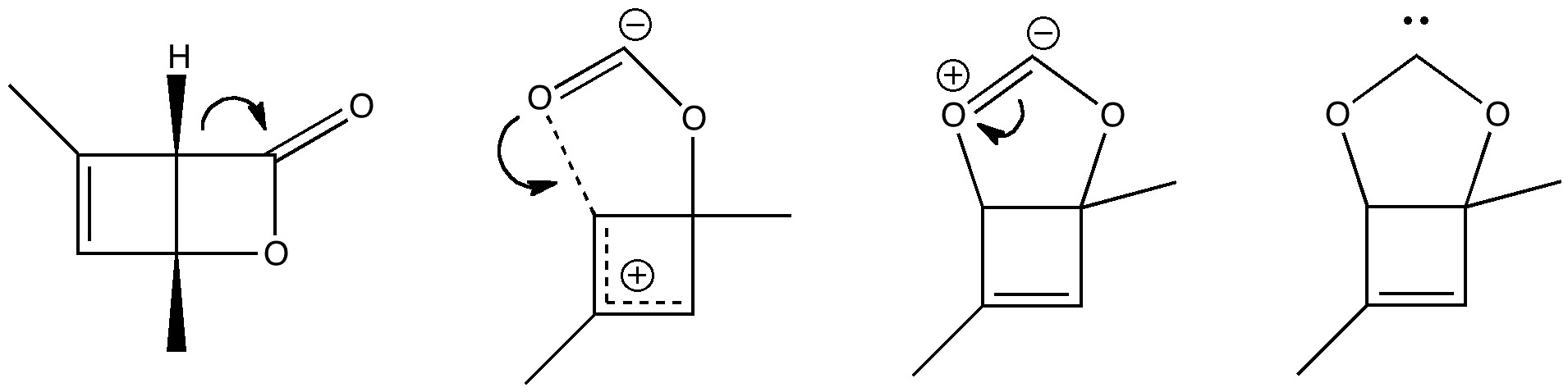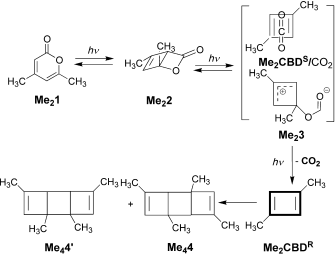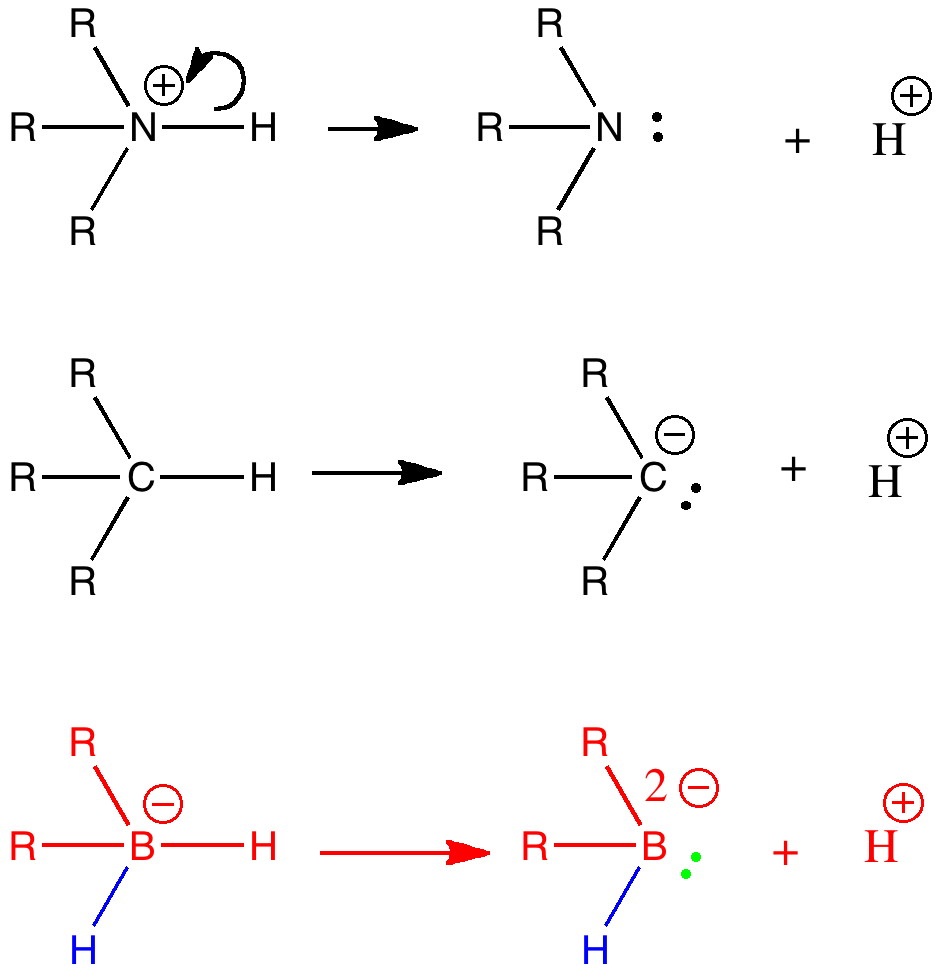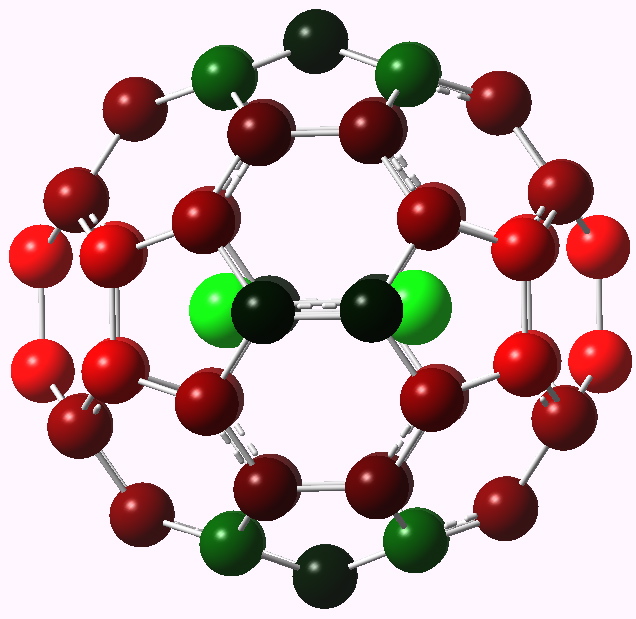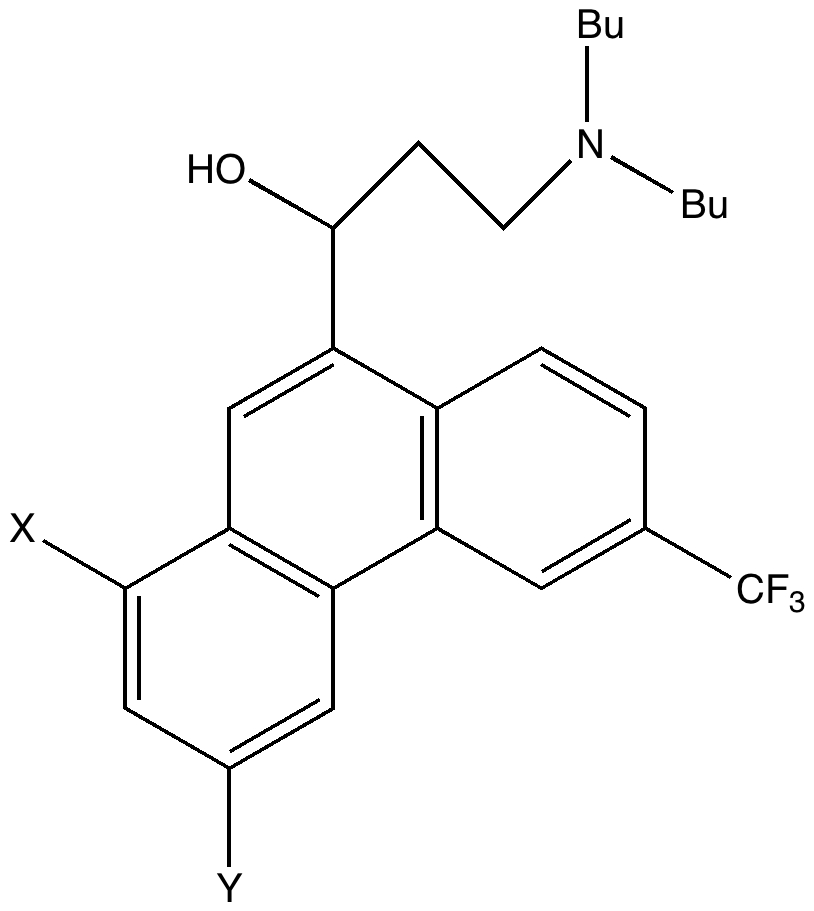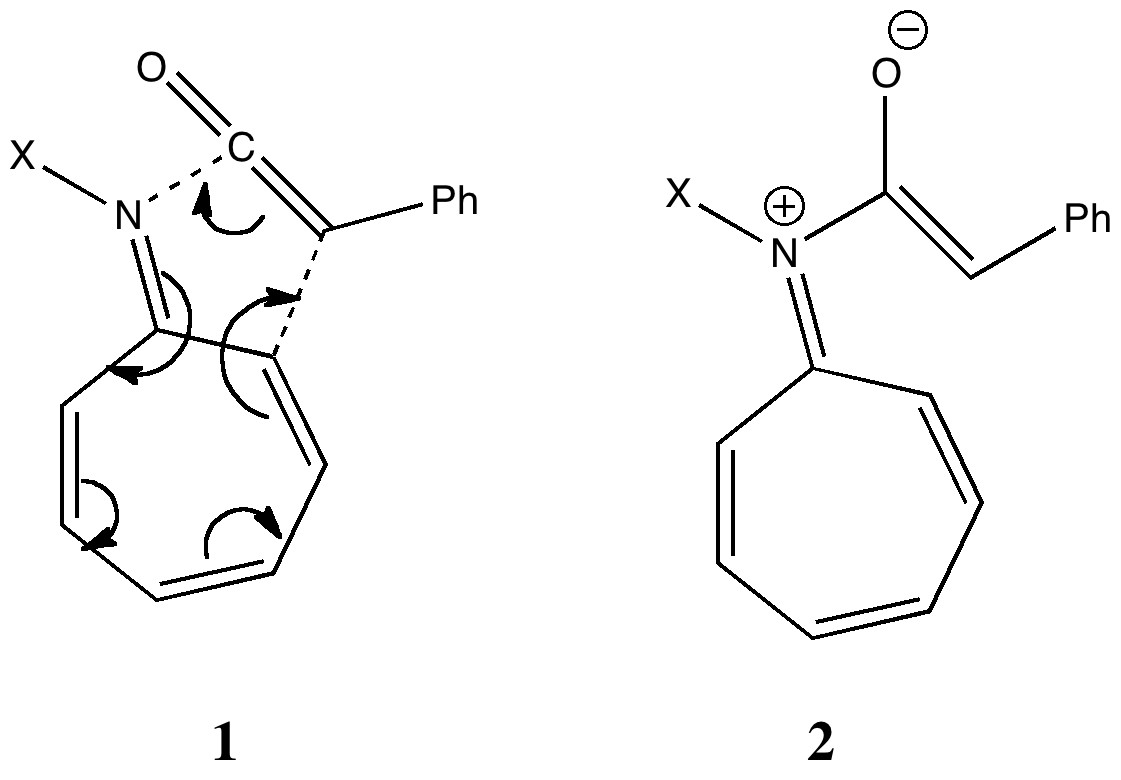
To (mis)quote Oscar Wilde again, ““To lose one methyl group may be regarded as a misfortune; to lose both looks like carelessness.” Here, I refer to the (past) tendency of molecular modellers to simplify molecular structures. Thus in 1977, quantum molecular modelling, even at the semi-empirical level, was beset by lost groups. One of my early efforts (DOI: 10.1021/ja00465a005) was selected for study because it had nothing left to lose;