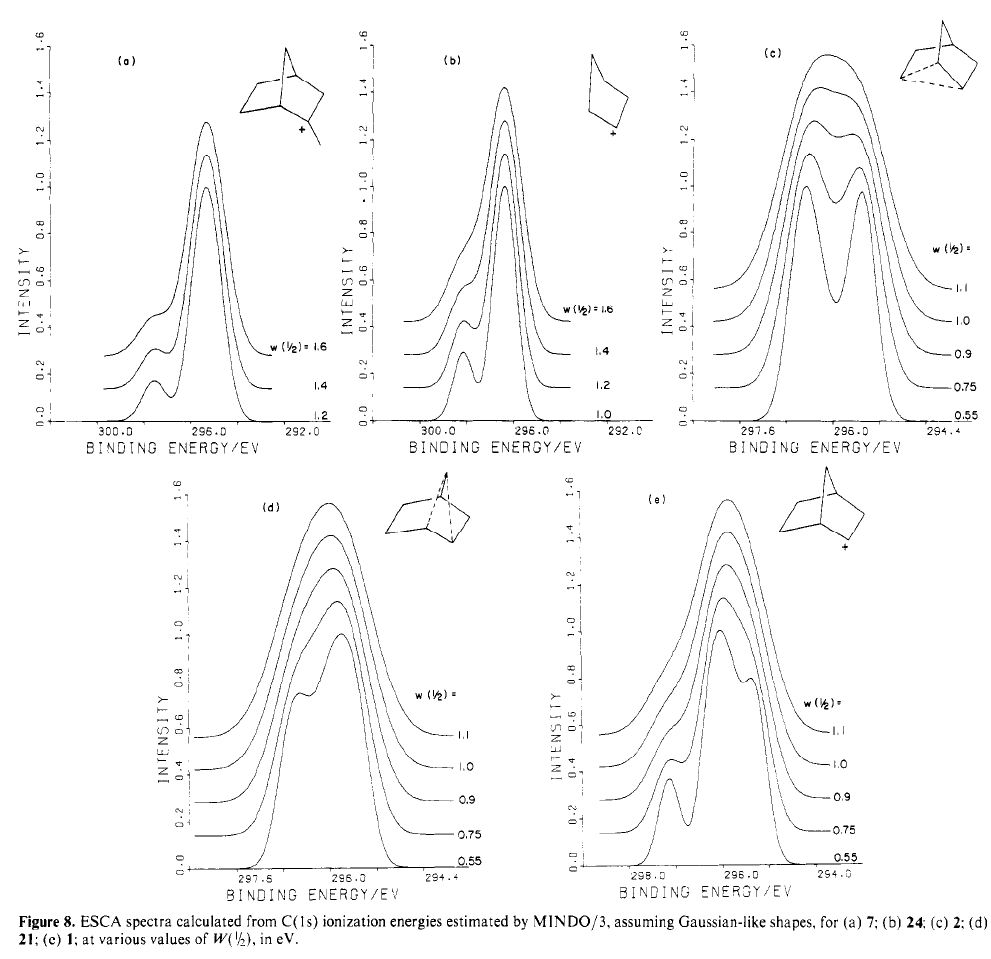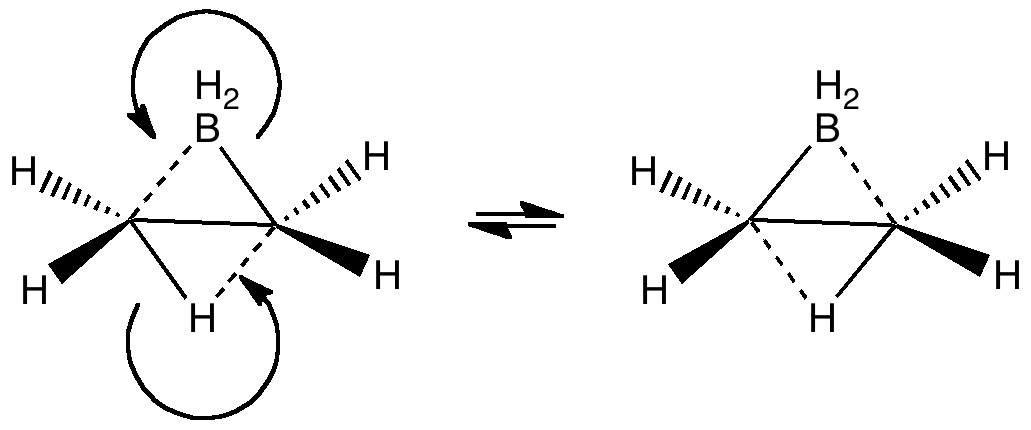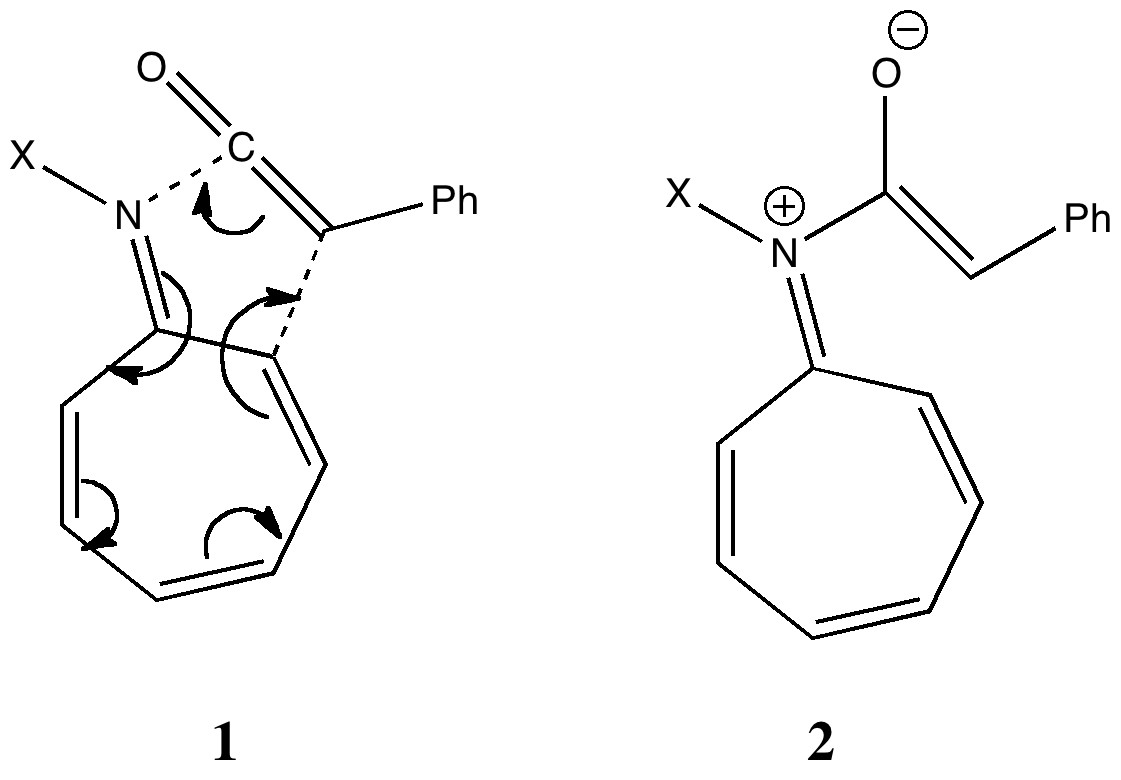
Steve Bachrach has blogged on the reaction shown below. If it were a pericyclic cycloaddition, both new bonds would form simultaneously, as shown with the indicated arrow pushing. Ten electrons would be involved, and in theory, the transition state would have 4n+2 aromaticity.