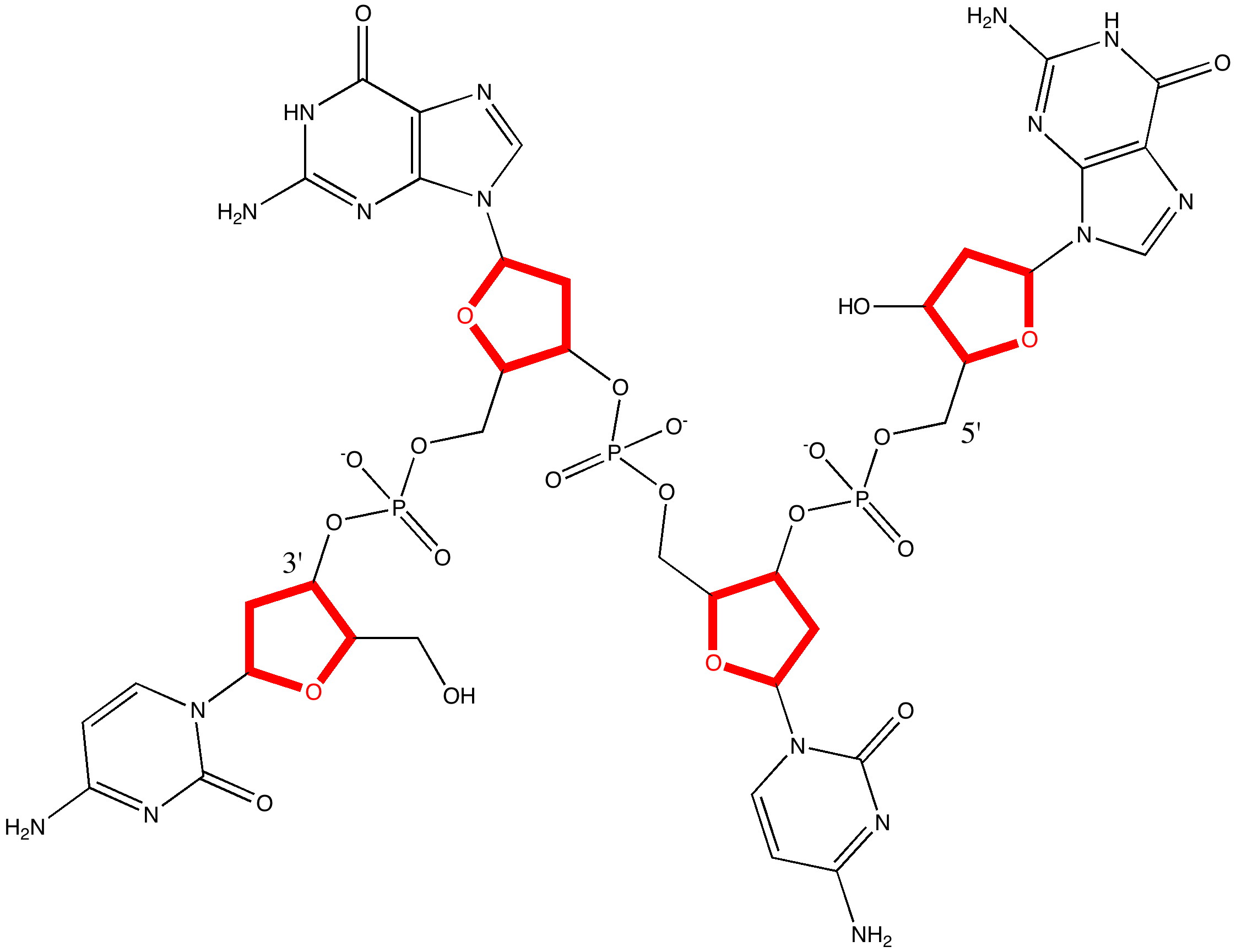
When Watson and Crick (WC) constructed their famous 3D model for DNA, they had to decide whether to make the double helix left or right handed.

When Watson and Crick (WC) constructed their famous 3D model for DNA, they had to decide whether to make the double helix left or right handed.

One of the delights of wandering around an undergraduate chemistry laboratory is discussing the unexpected, if not the outright impossible, with students. The >100% yield in a reaction is an example. This is sometimes encountered (albeit only briefly) when students attempt to recrystallise a product from cyclohexane, and get an abundant crop of crystals when they put their solution into an ice-bath to induce the crystallisation.

Science is about making connections. Plenty are on show in Watson and Crick’s famous 1953 article on the structure of DNA[cite]10.1038/171737a0[/cite] but often with the tersest of explanations. Take for example their statement “ Both chains follow right-handed helices ”. Where did that come from?
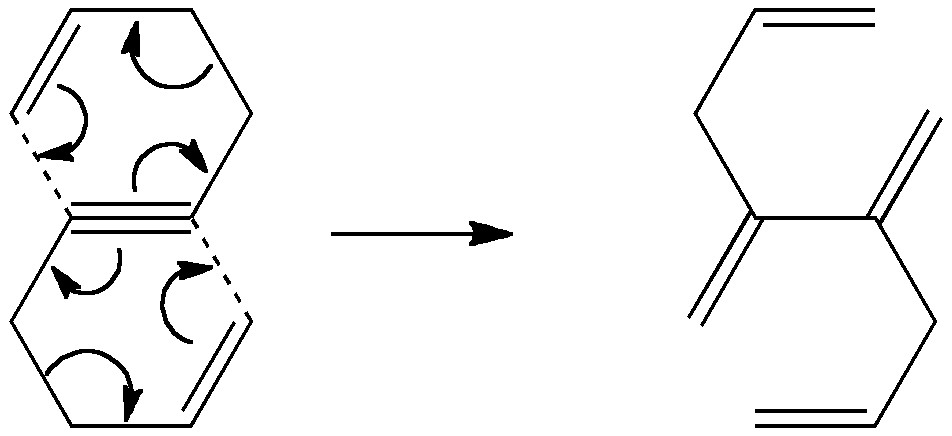
Is there a preferred pack size for electrons on the move? Or put less flamboyantly, is there an optimum, and a maximum number of arrows (electron pairs) that one might push in revealing the mechanism of a concerted reaction? A sort of village-instinct for electrons.
If you get a small rotatable molecule below, then ChemDoodle/HTML5/WebGL is working. Why might this be important? Well, the future is mobile, in other words, devices that rely on batteries or other sources of built-in power. This means the power guzzling GPU cards of the past (some reach ~400 Watts!) cannot be used.
If you visit this blog you will see a scientific discourse in action.

Chemistry gets complex very rapidly. Consider the formula CH 3 NO as the topic for a tutorial in introductory chemistry. I challenge my group (of about 8 students) to draw as many different molecules as they can using exactly those atoms. I imply that perhaps each of them might find a different structure; this normally brings disbelieving expressions to their faces.
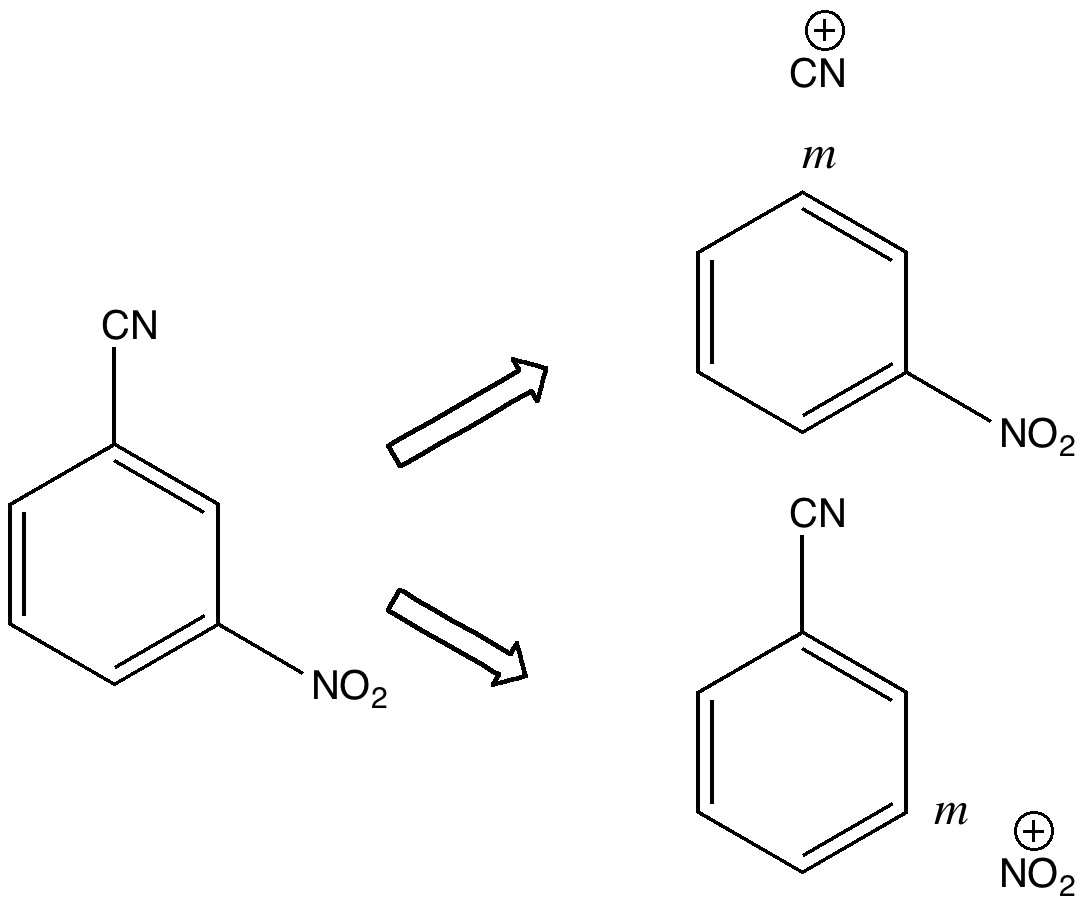
Do you fancy a story going from simplicity to complexity, if not absurdity, in three easy steps? Read on! The following problem appears in one of our (past) examination questions in introductory organic chemistry. From relatively mundane beginnings, one can rapidly find oneself in very unexpected territory. How would one make 3-nitrobenzonitrile?
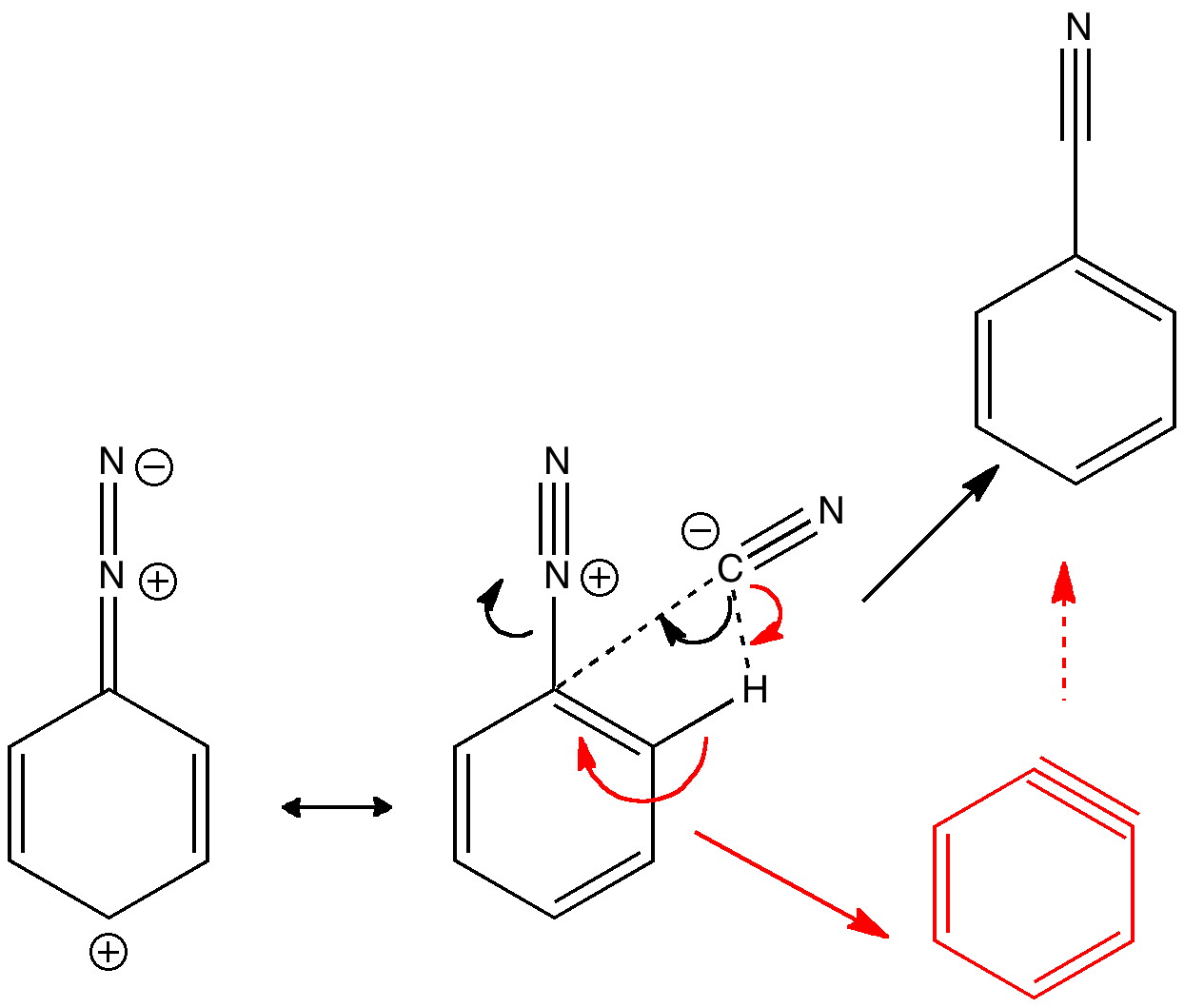
Janus was the mythological Roman god depicted as having two heads facing opposite directions, looking simultaneously into the past and the future. Some of the most ancient ( i.e. 19th century) known reactions can be considered part of a chemical mythology; perhaps it is time for a Janus-like look into their future. Reaction of the diazonium cation with cyanide.
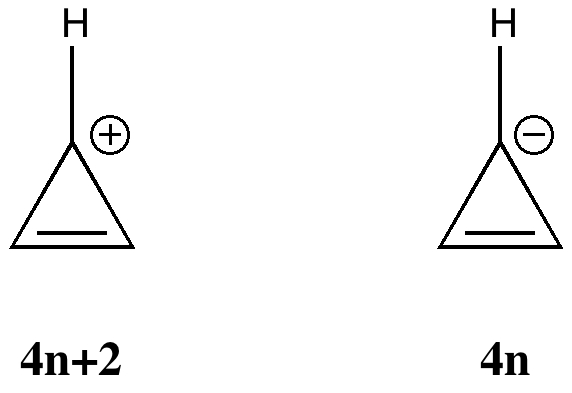
More inspiration from tutorials. In a lecture on organic aromaticity, the 4n+2/4n Hückel rule was introduced (in fact, neither rule appears to have actually been coined in this form by Hückel himself!). The simplest examples are respectively the cyclopropenyl cation and anion. The former has 2 π-electrons exhibiting cyclic delocalisation, and the 4n+2 (n=0) rule predicts aromaticity.
Moving (chemical) data around in a manner which allows its (automated) use in whichever context it finds itself must be a holy grail for all scientists and chemists.