Moving (chemical) data around in a manner which allows its (automated) use in whichever context it finds itself must be a holy grail for all scientists and chemists.

My university tutorial yesterday covered selective reductions of functional groups in organic chemistry. My thoughts on that topic have now morphed into something rather different. Scientific research has a habit of having this sort of thing happen.
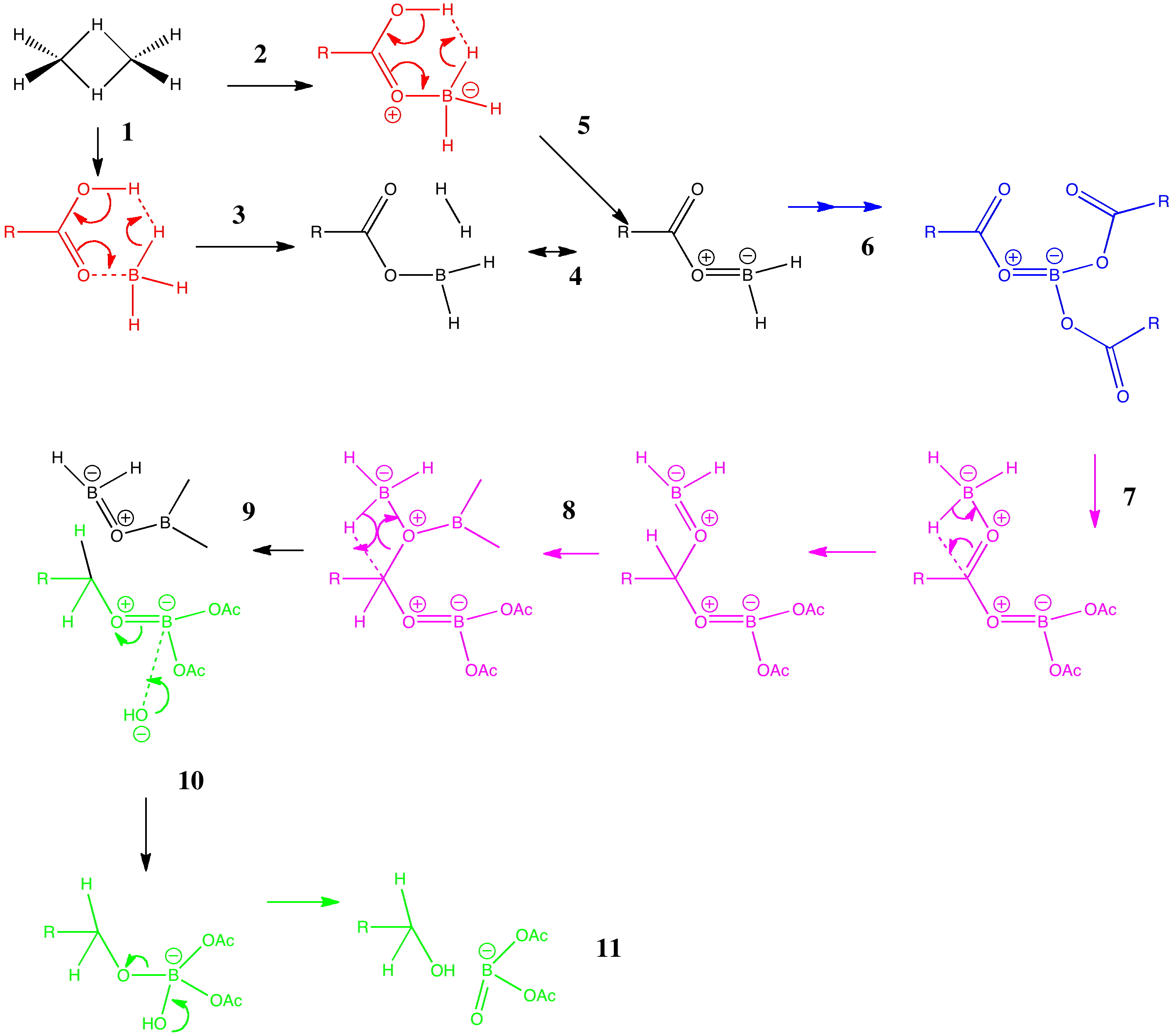
Arrow pushing (why never pulling?) is a technique learnt by all students of organic chemistry (inorganic chemistry seems exempt!). The rules are easily learnt (supposedly) and it can be used across a broad spectrum of mechanism. But, as one both becomes more experienced, and in time teaches the techniques oneself as a tutor, its subtle and nuanced character starts to dawn.
For those of us who were around in 1985, an important chemical IT innovation occurred. We could acquire a computer which could be used to draw chemical structures in one application, and via a mysterious and mostly invisible entity called the clipboard , paste it into a word processor (it was called a Macintosh). Perchance even print the result on a laserprinter.
Our understanding of science mostly advances in small incremental and nuanced steps (which can nevertheless be controversial) but sometimes the steps can be much larger jumps into the unknown, and hence potentially more controversial as well. More accurately, it might be e.g. relatively unexplored territory for say a chemist, but more familiar stomping ground for say a physicist.

On 8th August this year, I posted on a fascinating article that had just appeared in Science[cite]10.1126/science.1188002[/cite] in which the crystal structure was reported of two small molecules, 1,3-dimethyl cyclobutadiene and carbon dioxide, entrapped together inside a calixarene cavity.
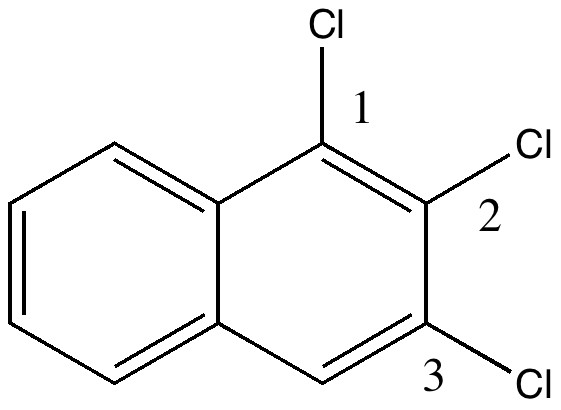
In 1890, chemists had to work hard to find out what the structures of their molecules were, given they had no access to the plethora of modern techniques we are used to in 2010. For example, how could they be sure what the structure of naphthalene was? Well, two such chemists, William Henry Armstrong (1847-1937) and his student William Palmer Wynne (1861-1950;
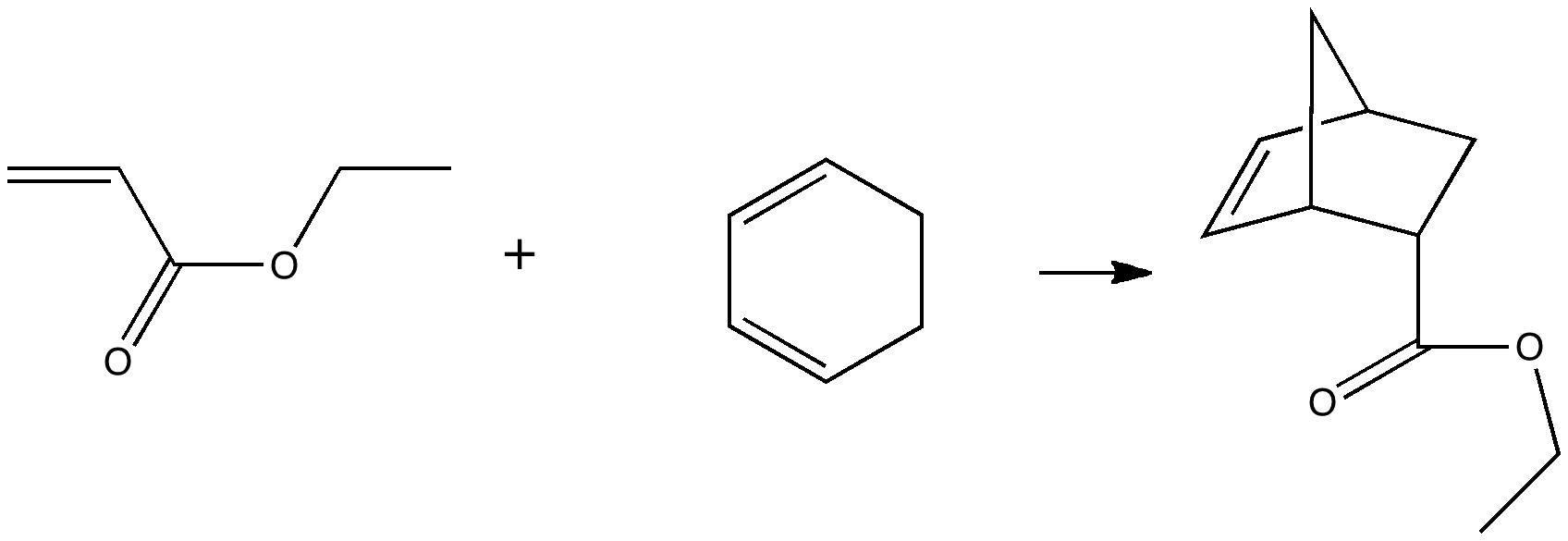
Reactions in cavities can adopt quite different characteristics from those in solvents. Thus first example of the catalysis of the Diels-Alder reaction inside an organic scaffold was reported by Endo, Koike, Sawaki, Hayashida, Masuda, and Aoyama[cite]10.1021/ja964198s[/cite], where the reaction shown below is speeded up very greatly in the presence of a crystalline lattice of the anthracene derivative shown below. A Diels-Alder reaction.

Curly arrows are something most students of chemistry meet fairly early on. They rapidly become hard-wired into the chemists brain. They are also uncontroversial! Or are they? Consider the following very simple scheme. Curly arrow pushing It represents protonation of an alkene by an acid.
The rather presumptious title assumes the laws and fundamental constants of physics are the same everywhere (they may not be). With this constraint (and without yet defining what is meant by strongest), consider the three molecules: Property (CCSD/aug-cc-pVTZ) N≡N (H-N≡N) + (H-N≡N-H) 2+ NN length, Å 1.0967 1.0915 1.0795 NN stretch, cm -1 2418.8 2356.4

One approach to reporting science which is perhaps better suited to the medium of a blog than a conventional journal article is the opportunity to follow ideas in unexpected, even unconventional directions. Thus my third attempt, like a dog worrying a bone, to explore hypervalency.