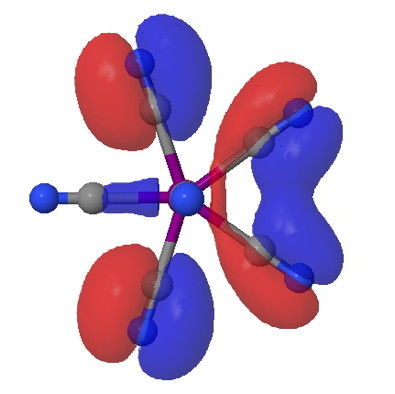
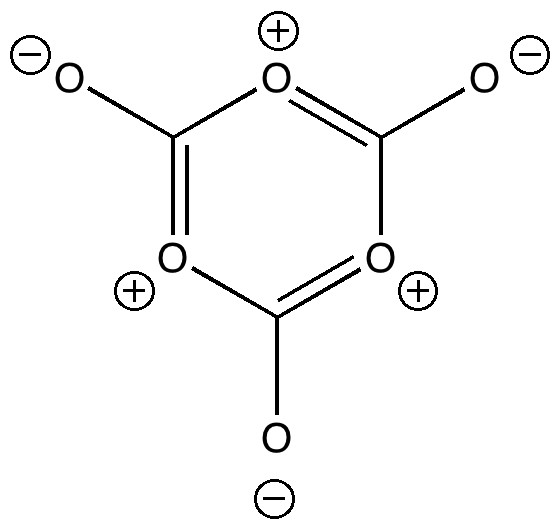
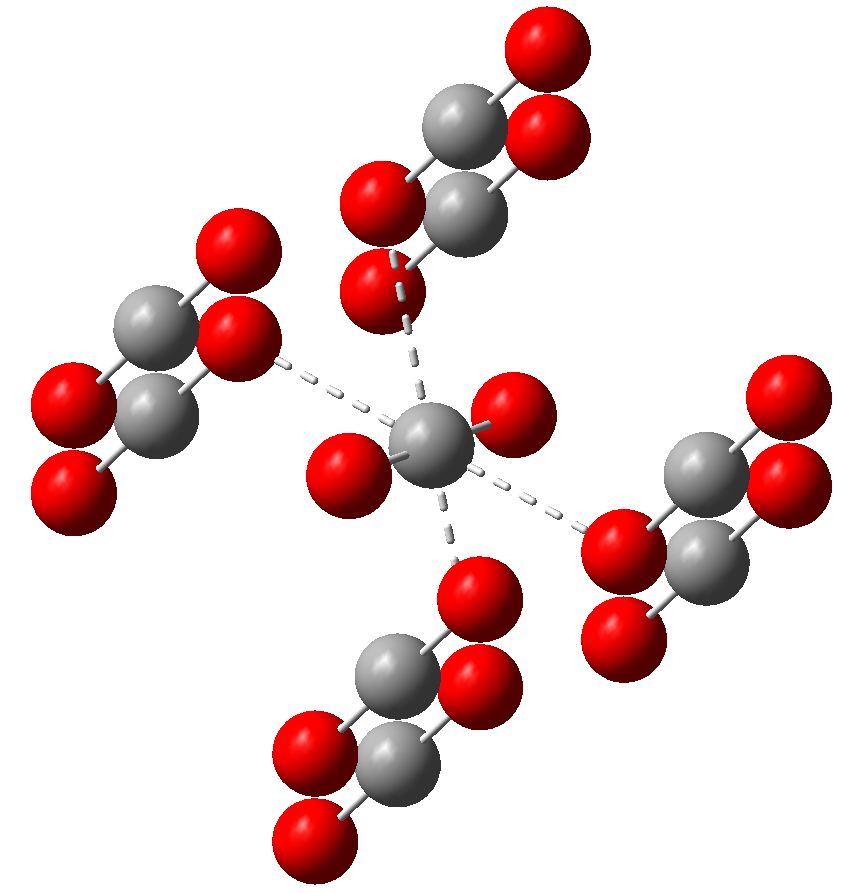
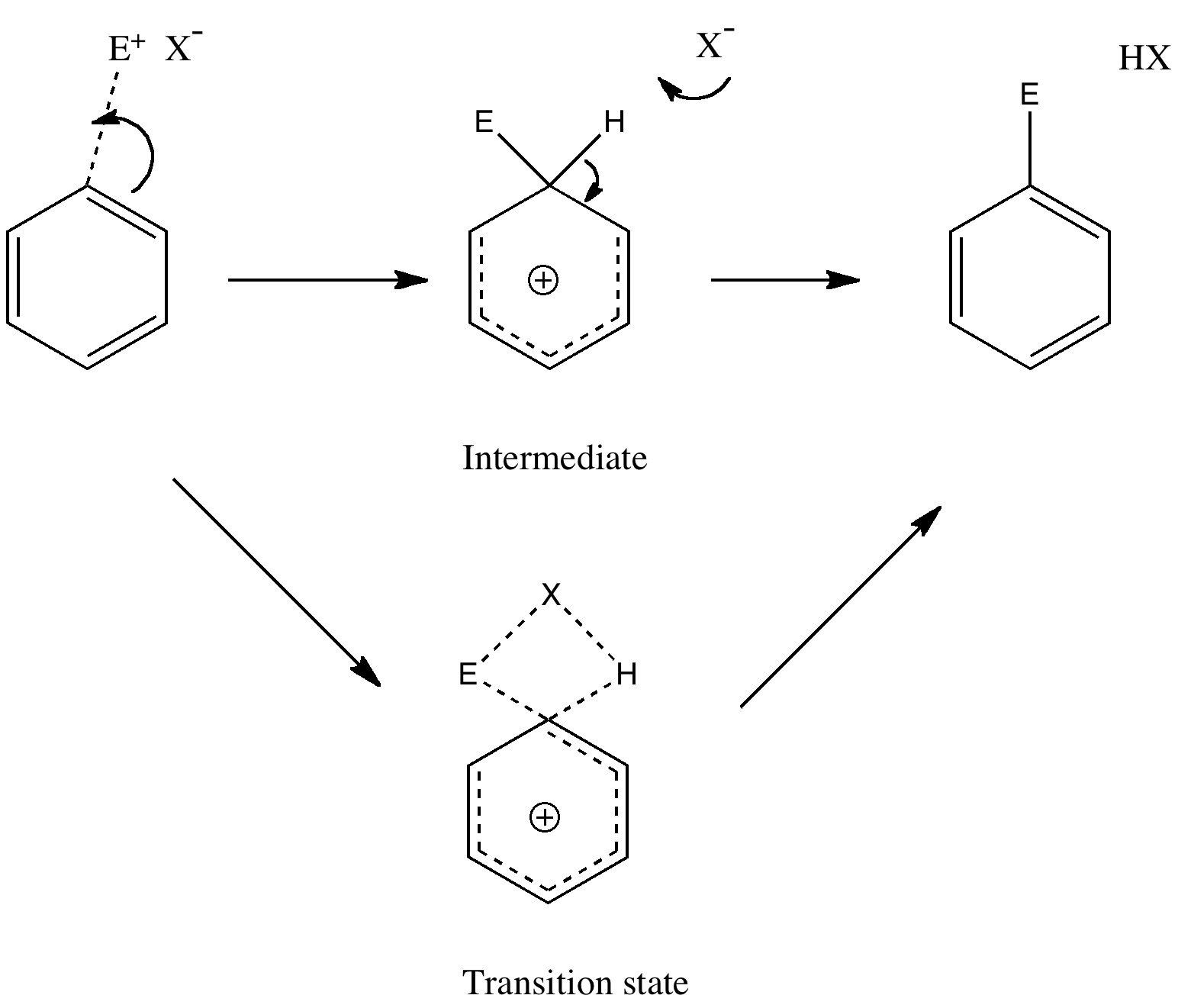
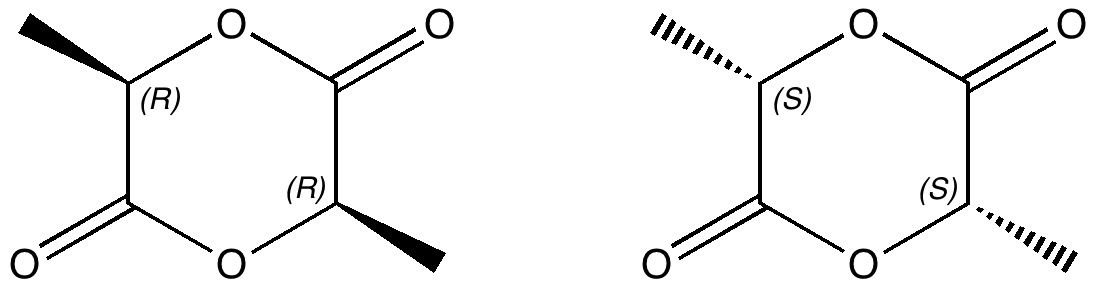
One approach to reporting science which is perhaps better suited to the medium of a blog than a conventional journal article is the opportunity to follow ideas in unexpected, even unconventional directions. Thus my third attempt, like a dog worrying a bone, to explore hypervalency.