Chemical bonds can be assembled from components which chemists know as σ, π and δ. The blog poses the question whether any bonds can be constructed which use a fourth type of component, the φ.

Chemical bonds can be assembled from components which chemists know as σ, π and δ. The blog poses the question whether any bonds can be constructed which use a fourth type of component, the φ.
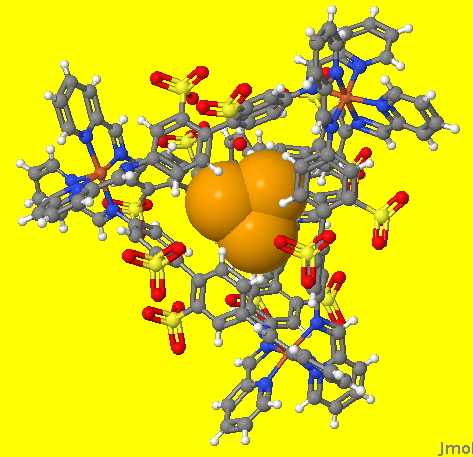
An earlier post described how a (spherical) halide anion fitted snugly into a cavity generated by the simple molecule propanone, itself assembled by sodium cations coordinating to the oxygen.

The iconic diagram below represents a cornerstone of organic chemistry.

This story starts with an organic chemistry tutorial, when a student asked for clarification of the Finkelstein reaction. This is a simple S N 2 type displacement of an alkyl chloride or bromide, using sodium iodide in acetone solution, and resulting in an alkyl iodide. What was the driving force for this reaction he asked?

In the previous post, it was noted that Möbius annulenes are intrinsically chiral, and should therefore in principle be capable of resolution into enantiomers. The synthesis of such an annulene by Herges and co-workers was a racemic one; no attempt was reported at any resolution into such enantiomers. Here theory can help, since calculating the optical rotation [α]D is nowadays a relatively reliable process for rigid molecules.

Much like climbing Mt. Everest because its there, some hypothetical molecules are just too tantalizing for chemists to resist attempting a synthesis. Thus in 1964, Edgar Heilbronner speculated on whether a conjugated annulene ring might be twistable into a Möbius strip. It was essentially a fun thing to try to do, rather than the effort being based on some anticipated (and useful) property it might have.
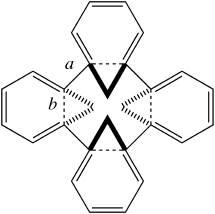
In 1988, Wilke[cite]10.1002/anie.198801851[/cite] reported molecule 1 It was a highly unexpected outcome of a nickel-catalyzed reaction and was described as a 24-annulene with an unusual 3D shape. Little attention has been paid to this molecule since its original report, but the focus has now returned!

The diagram below summarizes an interesting result recently reported by Hanson and co-workers (DOI: 10.1021/jo800706y. At ~neutral pH, compound 13 hydrolyses with a half life of 21 minutes, whereas 14 takes 840 minutes. Understanding this difference in reactivity may allow us to understand why some enzymes can catalyze the hydrolysis of peptides with an acceleration of up to twelve orders of magnitude.

Understanding how molecules interact (bind) with each other when in close proximity is essential in all areas of chemistry. One specific example of this need is for the molecule shown below. The Pirkle reagent This is the so-called Pirkle Reagent and is much used to help resolve the two enantiomers of a racemic mixture, particularly drug molecules.

Every introductory course or text on aromatic electrophilic substitution contains an explanation along the lines of the resonance diagram shown below. With an o / p directing group such as NH 2 , it is argued that negative charge accumulates in those positions as a result of the resonance structures shown.
We recently developed a new computational chemistry practical laboratory here at Imperial College. I gave a talk about it at the recent ACS meeting in Salt Lake City. If you want to see the details of the lab, do go here. The talk itself contains further links and examples.