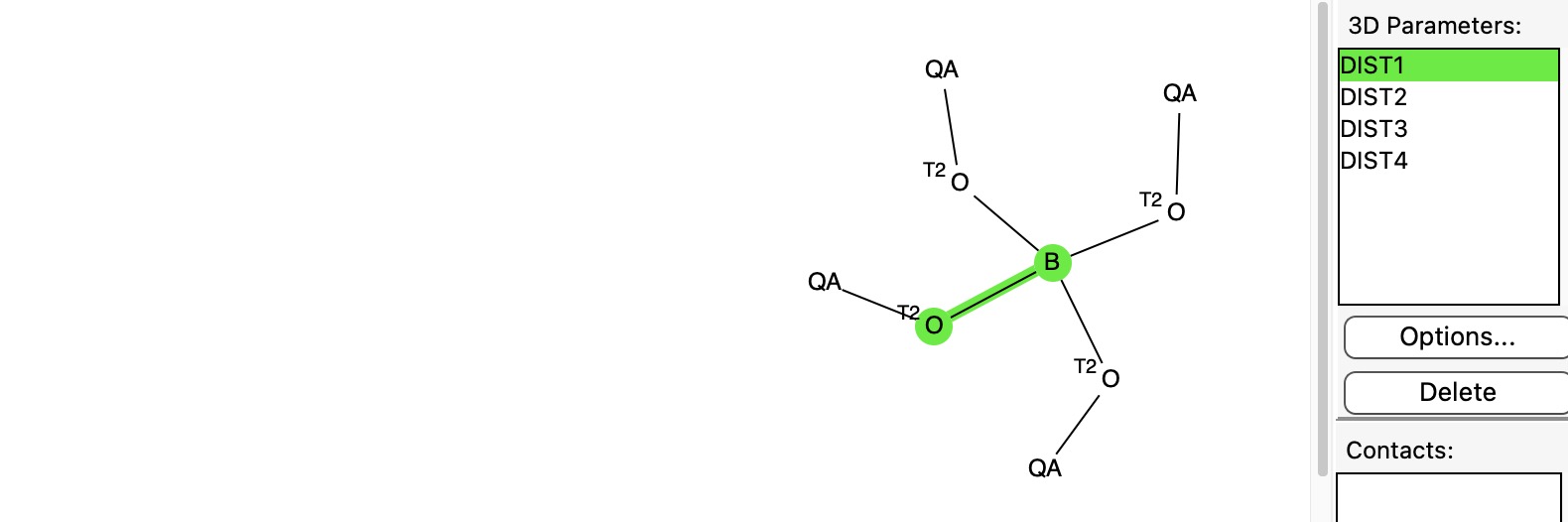
In an earlier post, I discussed[cite]10.59350/dfkt5-k2b20[/cite] a phenomenon known as the “anomeric effect” exhibited by tetrahedral carbon compounds with four C-O bonds. Each oxygen itself bears two bonds and has two lone pairs, and either of these can align with one of three other C-O bonds to generate an anomeric effect. Here I change the central carbon to a boron to explore what happens, as indeed I promised earlier.