Previously on this blog: modelling the reduction of cinnamaldehyde using one molecule of lithal shows easy reduction of the carbonyl but a high barrier at the next stage, the reduction of the double bond.
Last August, I wrote about data galore , the archival of data for 133,885 (134 kilo) molecules into a repository, together with an associated data descriptor[cite]10.1038/sdata.2014.22[/cite] published in the new journal Scientific Data . Since six months is a long time in the rapidly evolving field of RDM, or research data management, I offer an update in the form of some new observations.
The reduction of cinnamaldehyde by lithium aluminium hydride (LAH) was reported in a classic series of experiments[cite]10.1021/ja01197a060[/cite],[cite]10.1021/ja01202a082[/cite],[cite]10.1021/ja01190a082[/cite] dating from 1947-8. The reaction was first introduced into the organic chemistry laboratories here at Imperial College decades ago, vanished for a short period, and has recently been reintroduced again. ‡ The experiment is
This might be seen as cranking a handle by producing yet more examples of acids ionised by a small number of water molecules. I justify it (probably only to myself) as an exercise in how a scientist might approach a problem, and how it linearly develops with time, not necessarily in the directions first envisaged.

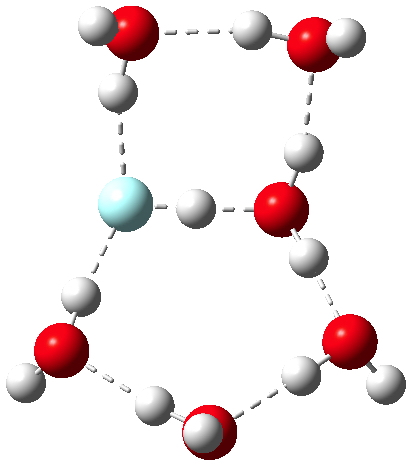
I do not play poker,‡ and so I had to look up a 5-4-3-2-1(A), which Wikipedia informs me is a 5-high straight flush, also apparently known as a steel wheel. In previous posts I have suggested acids which can be ionised by (probably) 5, 4, 3 or 1 discrete water molecules in the gas phase;

My previous posts have covered the ionization by a small number of discrete water molecules of the series of halogen acids, ranging from HI (the strongest, pKa -10) via HF (weaker, pKa 3.1) to the pseudo-halogen HCN (the weakest, pKa 9.2). Here I try out some even stronger acids to see what the least number of water molecule needed to ionize these might be. Firstly what must surely be the ultimate acid
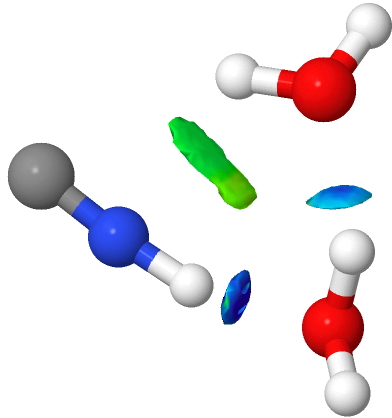
HCN is a weak acid (pKa +9.2, weaker than e.g. HF), although it does have an isomer, isocyanic acid or HNC (pka < +9.2 ?) which is simultaneously stronger and less stable. I conclude my halide acid series by investigating how many water molecules (in gas phase clusters) are required for ionisation of this “ pseudo-halogen ” acid.
Why is this post orphaned from the previous? In order to have the opportunity of noting that treating iodine computationally can be a little different from the procedures used for F, Cl and Br. As the nuclear charge increases proceeding down the periodic table, the inner electron shells start becoming relativistic.
No doubt answers to the question posed in the previous post are already being obtained by experiment. Just in case that does not emerge in the next day or so, I offer a prediction here. The methodology is the same as before, and I have not tried to look for new isomeric forms compared with the structures found with HCl.
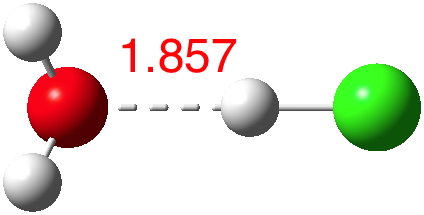
According to Guggemos, Slavicek and Kresin, about 5-6![cite]10.1103/PhysRevLett.114.043401[/cite]. This is one of those simple ideas, which is probably quite tough to do experimentally. It involved blasting water vapour through a pinhole, adding HCl and measuring the dipole-moment induced deflection by an electric field.
